Ligand-Controlled Regiodivergent Hydrosilylation of α,β-Unsaturated Esters
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Silyl-substituted esters have attracted considerable interest owing to their versatile reactivity. However, current methods typically use reactive organometallics and require multiple steps, with low atom economy and narrow substrate scope. These issues limit the utility of such compounds, especially those bearing hydrogen-containing silyl groups. Herein, we present a copper-catalyzed, regioselective hydrosilylation of α,β-unsaturated esters that enables efficient and tunable access to both α- and β-silyl esters bearing Si–H functionalities. In the presence of dppm as the ligand, undesired reduction pathways are effectively suppressed, affording α-silyl esters in high yields with excellent regioselectivity. Notably, this protocol is compatible with both dihydrosilanes and trihydrosilanes, marking the first example of α-silylation of α,β-unsaturated esters using trihydrosilanes. In contrast, employing dcype as the ligand promotes an anti-Michael-type addition, delivering β-silyl esters via an unusual α-addition of Cu–H to the α,β-unsaturated system. Deuterium-labeling studies support the involvement of a distinct α-addition mechanism. Density functional theory (DFT) calculations pinpointed the stabilizing role of the weak C–H···π interaction between the dcype ligand and the aryl ring of the substrate in the regioselectivity reversed racemic β-silylation that reversed the trend to slightly favor the β-selectivity, a trend that is augmented by solvent and temperature effects.
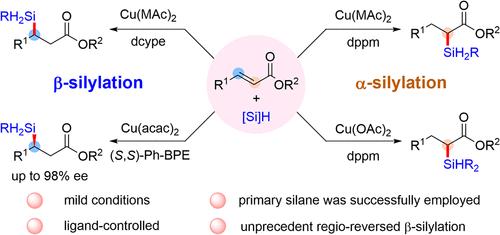
配体控制的α,β-不饱和酯的区域发散型硅氢化反应
硅基取代酯由于其多用途反应性而引起了人们的极大兴趣。然而,目前的方法通常使用反应性有机金属,需要多个步骤,原子经济性低,衬底范围窄。这些问题限制了这类化合物的使用,特别是那些含氢硅基的化合物。在这里,我们提出了一种铜催化的,区域选择性的α,β-不饱和酯的硅氢化反应,使α-和β-硅基酯具有Si-H官能团的有效和可调的途径。在dppm作为配体存在的情况下,不需要的还原途径被有效抑制,提供高收率的α-硅基酯,具有优异的区域选择性。值得注意的是,该方案兼容于二氢硅烷和三氢硅烷,标志着用三氢硅烷对α,β-不饱和酯进行α-硅化的第一个例子。相反,采用型作为配体促进了反迈克尔型加成,通过在α,β-不饱和体系中不寻常的α- Cu-H加成传递β-硅基酯。氘标记研究支持α-加成机制的参与。密度泛函理论(DFT)计算指出,型配体与底物芳基环之间的弱C-H··π相互作用在区域选择性逆转的外消旋β-硅基化过程中起稳定作用,使β-硅基化趋势略微偏向于选择性,溶剂和温度的影响增强了这一趋势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


