The Biogeochemical Cycling of Zinc in Terrestrial Ecosystems: Insights from Zinc Isotopes
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Zinc (Zn) isotope geochemistry is a powerful tool for tracing Zn biogeochemical cycling in terrestrial ecosystems. This review comprehensively synthesizes Zn isotope signatures and fractionation mechanisms in atmospheric, soil, aquatic, and plant systems, integrating laboratory experiments and field observations. Laboratory studies have identified key drivers of Zn isotope fractionation, including mineral dissolution-precipitation, adsorption onto mineral surfaces, and organic complexation. Field studies show that interactions, particularly the competition between OM and (hydr)oxides, dominate Zn isotope compositions, with OM-rich soils exhibiting relatively lower δ66Zn values. Anthropogenic activities significantly influence Zn isotope signatures. Agricultural practices exert an insignificant effect on natural δ66Zn; traffic emissions exhibit slightly light Zn isotope compositions; urban emissions (e.g., electroplating) and industrial processes (e.g., smelting) are the predominant Zn sources, inducing significant Zn isotopic offsets. Plants preferentially uptake 64Zn, while selective retention of 66Zn in root cell walls may cover the uptake preference. Furthermore, translocation within plants occurs through low-affinity (diffusion-driven) and high-affinity (carrier protein-mediated) pathways, favoring 64Zn and 66Zn, respectively. These mechanisms collectively govern Zn isotopic variations. Despite progress, key knowledge gaps remain, particularly in quantifying intracellular isotope fractionation and disentangling multi-process interactions under natural conditions. Future research should integrate multi-isotope tracers (e.g., Zn-Cd-Pb), spatially resolved microanalytical techniques, and predictive modeling to refine pollution tracing and advance mechanistic understanding of Zn cycling. By bridging laboratory insights with ecosystem-scale observations, this review highlights the potential of Zn isotopes to address environmental challenges, from tracing metal pollution to guiding sustainable resource management in the Anthropocene.
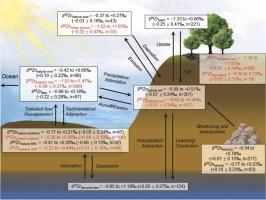
锌在陆地生态系统中的生物地球化学循环:来自锌同位素的见解
锌同位素地球化学是追踪陆地生态系统锌生物地球化学循环的有力工具。本文结合室内实验和野外观测,对Zn在大气、土壤、水生和植物系统中的同位素特征及其分馏机制进行了综合评述。实验室研究已经确定了锌同位素分馏的关键驱动因素,包括矿物溶解沉淀、矿物表面吸附和有机络合作用。野外研究表明,锌同位素组成主要是相互作用,特别是OM和(氢氧)氧化物之间的竞争,富OM土壤的δ66Zn值相对较低。人类活动显著影响Zn同位素特征。农业实践对天然δ66Zn的影响不显著;交通排放的Zn同位素组成略轻;城市排放(如电镀)和工业过程(如冶炼)是主要的锌源,引起显著的锌同位素抵消。植物优先吸收64Zn,而66Zn在根细胞壁的选择性保留可能掩盖了吸收偏好。此外,植物内部的易位通过低亲和力(扩散驱动)和高亲和力(载体蛋白介导)途径进行,分别倾向于64Zn和66Zn。这些机制共同支配着锌同位素的变化。尽管取得了进展,但关键的知识差距仍然存在,特别是在定量细胞内同位素分拣和解开自然条件下多过程相互作用方面。未来的研究应结合多同位素示踪剂(如Zn- cd - pb)、空间分辨微分析技术和预测模型,以完善污染追踪和推进对Zn循环机制的理解。通过将实验室见解与生态系统规模的观测相结合,本综述强调了锌同位素在解决环境挑战方面的潜力,从追踪金属污染到指导人类世的可持续资源管理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


