Ionic Liquid Electrolyte Suppresses Deep Sodiation in Nb4P2S21/Mo2CTx Enabling Transition from Mixed-Voltage to Pure High-Voltage Operation for Sodium-Ion Battery Cathodes
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Elemental sulfur has garnered significant attention due to its low cost and high theoretical capacity; however, its reliance on ether electrolytes leads to the formation of soluble polysulfides, thereby limiting its application. Sulfur-rich transition metal polysulfides demonstrate potential as sulfur-equivalent cathodes to replace conventional sulfur in alkali metal–sulfur batteries; however, adequate research in this area remains unrevealed. In this study, we investigate the Nb4P2S21 in carbonate, ether, and ionic liquid electrolytes for sodium-ion battery testing. The material exhibits a high discharge capacity exceeding 1000 mAh/g and a prolonged discharge plateau at low potentials in both ether and carbonate electrolytes, same with other high-capacity phosphorus sulfide anodes via conversion reactions. When switching to the NaTFSI/[Emim]TFSI ionic liquid electrolyte, 96.3% of the initial discharge capacity in the 0–3 V range is retained above 0.8 V, with the suppression of low-voltage redox activity. This shift is attributed to the cointercalation of Na+ and Emim+ ions, preventing the materials from deep sodiation at lower voltage range. The incorporation of Mo2CTx MXene into the material further reduces electrochemical polarization and enhances cycle stability. During 100 cycles, a self-activation phenomenon occurs, resulting in a maximum capacity of 384 mAh/g, while the median voltage remains above 1.5 V, predominantly governed by a pair of reversible redox peaks. X-ray photoelectron spectroscopy (XPS) and high-resolution transmission electron microscopy (HRTEM) analyses of postcycled material confirm the structural and compositional stability of the material during cycling. This study advances the understanding of sulfur-rich materials in sodium-ion batteries across various electrolytes, particularly ionic liquids.
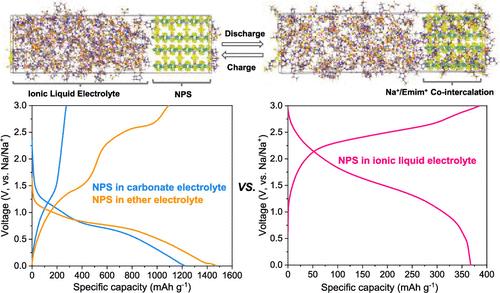
离子液体电解质抑制Nb4P2S21/Mo2CTx中的深度钠化,使钠离子电池阴极从混合电压过渡到纯高压工作
单质硫因其低成本和高理论容量而受到广泛关注;然而,它对醚电解质的依赖导致了可溶性多硫化物的形成,从而限制了它的应用。富硫过渡金属多硫化物显示出在碱金属-硫电池中作为硫当量阴极替代传统硫的潜力;然而,在这方面的充分研究仍未公布。在这项研究中,我们研究了Nb4P2S21在碳酸盐,醚和离子液体电解质中的钠离子电池测试。该材料在乙醚和碳酸盐电解质中具有超过1000 mAh/g的高放电容量,并且在低电位下具有较长的放电平台,与其他高容量硫化磷阳极通过转化反应相同。切换到NaTFSI/[Emim]TFSI离子液体电解质时,0.8 V以上0-3 V范围内的初始放电容量保留96.3%,低压氧化还原活性受到抑制。这种转变归因于Na+和Emim+离子的共插层,阻止了材料在较低电压范围内的深度硫化。在材料中掺入Mo2CTx MXene进一步降低了电化学极化,提高了循环稳定性。在100次循环中,发生自激活现象,导致最大容量为384 mAh/g,而中位电压保持在1.5 V以上,主要由一对可逆氧化还原峰控制。循环后材料的x射线光电子能谱(XPS)和高分辨率透射电子显微镜(HRTEM)分析证实了材料在循环过程中的结构和成分稳定性。这项研究促进了对钠离子电池中各种电解质,特别是离子液体中富硫材料的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


