Enhanced Stability of Vanadium-Based Electrode Materials Using Multi-Component Hybrids for High-Performance Zinc-Ion Batteries
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
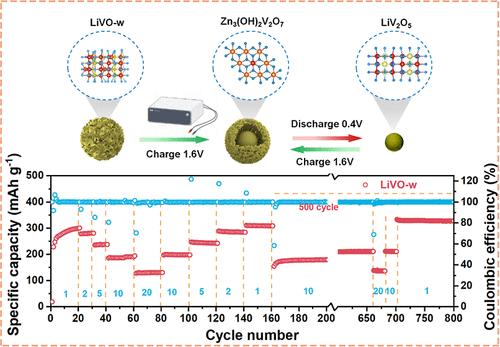
The limited availability of cathode materials for rechargeable aqueous zinc-ion batteries (ZIBs), which have great potential for grid-scale energy storage applications, remains a significant obstacle to development. In this study, we proposed a Li3VO4–LiV2O5-based (LiVO-w) nanocomposite structure obtained by simple high-temperature calcination and water washing as a high-performance cathode. The LiVO-w cathode demonstrates a high specific capacity (310.43 mAh g–1 at 1 A g–1 and 130.52 mAh g–1 at 20 A g–1) and excellent cycling stability (100% capacity retention after 4000 cycles at 10 A g–1 and 85.13% capacity retention after 10,000 cycles). In addition, ex situ X-ray diffraction (XRD) shows the structural transformation of LiVO-w during the self-assembly process. During the first charge process, the multivalent and multistructured LiVO-w undergoes an increase in the valence of V, accompanied by the generation of Zn3(OH)2V2O7·H2O on the surface of the matrix. The charge and discharge process after self-assembly mainly corresponds to the generation and decomposition of Zn3(OH)2V2O7·H2O. This excellent self-assembled matrix realizes the realization of LiVO-w cathodes with high capacity and high-capacity retention, representing a major advancement in the commercial development of ZIBs for the development of LiVO-w positive electrode materials.
采用多组分混合材料增强高性能锌离子电池钒基电极材料的稳定性
可充电水性锌离子电池(zib)具有巨大的电网储能应用潜力,但其正极材料的有限性仍然是其发展的一个重大障碍。在这项研究中,我们提出了一种基于li3vo4 - liv2o5 (LiVO-w)的纳米复合结构,通过简单的高温煅烧和水洗得到作为高性能阴极。LiVO-w阴极具有很高的比容量(1a g-1时310.43 mAh g-1, 20a g-1时130.52 mAh g-1)和优异的循环稳定性(在10a g-1下4000次循环后容量保持100%,10000次循环后容量保持85.13%)。此外,非原位x射线衍射(XRD)显示了自组装过程中LiVO-w的结构转变。在第一次充电过程中,多价多结构的LiVO-w经历了V价的升高,同时在基体表面生成了Zn3(OH)2V2O7·H2O。自组装后的充放电过程主要对应于Zn3(OH)2V2O7·H2O的生成和分解。这种优异的自组装矩阵实现了高容量和高容量保留的LiVO-w阴极,代表了ZIBs商业化开发LiVO-w正极材料的重大进步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


