HFIP/BF3·Et2O Synergistic Catalysis Enables Intramolecular Alkyne Cyclization to Coumarins
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We have successfully developed a novel synergistic catalytic system employing hexafluoroisopropanol (HFIP)/BF3·Et2O for intramolecular alkyne cyclization, which enables efficient construction of coumarin frameworks. This metal-free protocol features mild reaction conditions and exceptional substrate generality. The methodology has been directly applied to the synthesis of pharmacologically important natural products and drug molecules. Systematic mechanistic investigations have uncovered the cooperative catalytic mechanism of HFIP in this transformation, providing new fundamental principles for reaction design.
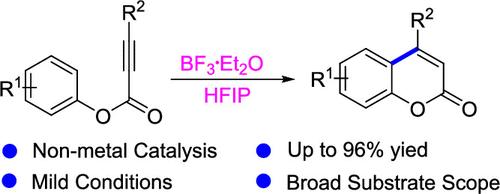
HFIP/BF3·Et2O协同催化实现香豆素分子内炔环化。
我们成功开发了一种采用六氟异丙醇(HFIP)/BF3·Et2O进行分子内炔环化的新型协同催化体系,可以高效地构建香豆素框架。这种无金属方案具有温和的反应条件和特殊的底物普遍性。该方法已直接应用于重要药理学天然产物和药物分子的合成。系统的机理研究揭示了HFIP在这一转化过程中的协同催化机理,为反应设计提供了新的基本原理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


