Isothermal nucleic acid amplification-based magnetic aptasensor for sensitive detection and in situ imaging of Trop-2 in triple-negative breast cancer
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
Triple-negative breast cancer (TNBC) is strongly linked to overexpression of Trop-2, a transmembrane glycoprotein biomarker implicated in its aggressive behavior. For in situ imaging and quantification of this membrane protein, we developed a highly sensitive fluorescence aptasensor.
Results
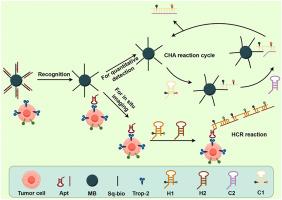
Upon recognition of Trop-2 by its specific aptamer, hybridization chain reaction (HCR) was triggered for in situ imaging of cell-surface Trop-2. Concurrently, catalytic hairpin assembly (CHA) amplified fluorescence signals for quantitative Trop-2 detection. Successful cellular imaging and quantitative Trop-2 detection were achieved a linear response was observed from 0.001 μM to 0.1 μM, the limit of detection (LOD) was determined to be 0.0009 μM. Trop-2 surface density measurements revealed average amount per cell of: 6.0 × 106 molecules per cell (MDA-MB-468), 2.0 × 106 molecules per cell (MCF-7), 6.6 × 105 molecules per cell (MDA-MB-231), and 4.1 × 105 molecules per cell (HL-7702).
Significance
To our current knowledge, the work represents the first aptasensor enabling both in situ Trop-2 protein imaging and quantitative comparison across diverse cell types. The proposed method shows promise for tumor diagnosis and therapeutic evaluation.

基于等温核酸扩增的磁偶体传感器用于三阴性乳腺癌中Trop-2的敏感检测和原位成像
背景三阴性乳腺癌(TNBC)与Trop-2的过度表达密切相关,Trop-2是一种跨膜糖蛋白生物标志物,与其侵袭性行为有关。为了对这种膜蛋白进行原位成像和定量,我们开发了一种高灵敏度的荧光配体传感器。结果在Trop-2被其特异性适配体识别后,触发杂交链反应(HCR)对细胞表面Trop-2进行原位成像。同时,催化发夹组装(CHA)扩增荧光信号用于定量检测Trop-2。在0.001 μM ~ 0.1 μM范围内观察到线性响应,检测限(LOD)为0.0009 μM。Trop-2表面密度测量显示每个细胞的平均含量为:6.0 × 106分子/细胞(MDA-MB-468), 2.0 × 106分子/细胞(MCF-7), 6.6 × 105分子/细胞(MDA-MB-231)和4.1 × 105分子/细胞(HL-7702)。据我们目前所知,这项工作代表了第一个能够在不同细胞类型中进行原位Trop-2蛋白成像和定量比较的适体传感器。该方法有望用于肿瘤诊断和治疗评价。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


