A dual PROTAC nanocarrier amplifies DNA damage and STING activation for cancer immunotherapy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The cytoplasmic accumulation of damaged DNA to activate the STING pathway has emerged as a promising strategy to enhance tumor immunogenicity and improve the efficacy of immune checkpoint blockade (ICB) therapy. Herein, a stimuli-responsive and dual PROTAC-embedded immunoactivator (denoted as Sd@Lip) is developed to potentiate ICB through amplified DNA damage and robust STING activation. Sd@Lip comprises an acid-sensitive liposomal nanocarrier co-encapsulating two targeted degraders of dBET1 (a BRD4 degrader) and SK-575 (a PARP1 degrader), which enhances drug solubility, stability, and enable precise stoichiometric co-delivery. Mechanistically, Sd@Lip enhances DNA damage by simultaneously disrupting both nonhomologous end joining (NHEJ) and homologous recombination (HR) repair pathways, leading to cytoplasmic DNA accumulation that activates STING signaling, induces proinflammatory cytokine release and enhances infiltration of immune effector cells. This immunogenic cascade promotes the recruitment of natural killer (NK) cells and cytotoxic T lymphocytes into the tumor microenvironment, thereby significantly augmenting the therapeutic efficacy of ICB against both primary and metastatic breast tumors. Collectively, this study highlights a synergistic PARP1 and BRD4 degradation strategy to induce immunostimulatory DNA damage, offering a compelling approach to improve outcomes in breast cancer immunotherapy.
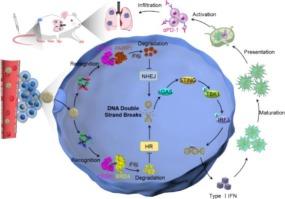
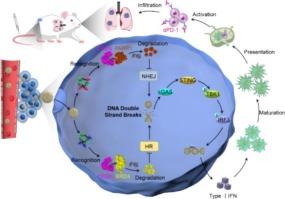
双PROTAC纳米载体放大DNA损伤和STING激活用于癌症免疫治疗
受损DNA的细胞质积累激活STING通路已成为增强肿瘤免疫原性和提高免疫检查点阻断(ICB)治疗效果的一种有希望的策略。本文开发了一种刺激反应性和双protac嵌入的免疫激活剂(表示为Sd@Lip),通过扩增DNA损伤和强大的STING激活来增强ICB。Sd@Lip是一种酸敏感脂质体纳米载体,共包封了两种靶向降解物dBET1 (BRD4降解物)和SK-575 (PARP1降解物),增强了药物的溶解度、稳定性,并实现了精确的化学计量共递送。从机制上说,Sd@Lip通过同时破坏非同源末端连接(NHEJ)和同源重组(HR)修复途径来增强DNA损伤,导致细胞质DNA积累,激活STING信号,诱导促炎细胞因子释放,增强免疫效应细胞的浸润。这种免疫原级联促进自然杀伤细胞(NK)和细胞毒性T淋巴细胞募集到肿瘤微环境中,从而显著增强了ICB对原发性和转移性乳腺肿瘤的治疗效果。总的来说,这项研究强调了PARP1和BRD4降解的协同策略,以诱导免疫刺激性DNA损伤,为改善乳腺癌免疫治疗的结果提供了一种令人信服的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


