Is it possible to valorize bicarbonates from reclaimed wastewater by CO2 electroreduction into formic acid? Investigation under low bicarbonate concentration and low-conductivity solutions
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
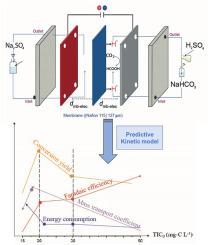
The electroreduction of CO2 into value-added compounds from low bicarbonate concentration solutions typical of treated wastewater has been explored for the first time, unlike existing studies that focus on high bicarbonates concentrations and pressurized gas CO2 as sources. This study investigates the feasibility of producing formic acid (FA) from low initial total inorganic carbon (TIC0) concentration referring to total inorganic carbon (TIC) after acidification to pH 4 (15-50 mg-C L−1) under microfluidic conditions in a filter press cell, from simulated to real reclaimed wastewaters. The effect of TIC0 demonstrated that an optimal TIC0 of 20 mg-C L−1 at japp = 5 mA cm−2 balanced the conversion yield (56 %), faradaic efficiency (FE) (9 %), and specific energy consumption (Esp) (1.96 kWh mol CO2−1). Reducing the japp from 5 to 2 mA cm−2 resulted in a 14 % increase in FE, although accompanied by a decrease in an overall conversion efficiency, highlighting the compromise that exists between selectivity and productivity. A kinetic model based on TIC degradation and FA formation accurately captured experimental trends. Results from real effluents showed successful FA production.
These findings highlighted the interdependence between mass transport (km) limitations, TIC availability, and competing reactions such as hydrogen evolution reaction (HER). The observed variations in km (1.4–1.6 × 10−5 m s−1) indicated that mass transport constraints played a critical role in CO2 reduction efficiency, where an increased diffusion layer () at higher TIC0 limited CO2 availability to the cathode surface shifting the balance between CO2 reduction and HER. FTIR analyses further revealed an intensification of FA bands with increasing current.
是否有可能通过CO2电还原将再生废水中的碳酸氢盐转化为甲酸?低碳酸氢盐浓度和低电导率溶液下的研究。
与现有的研究不同,现有的研究主要集中在高碳酸氢盐浓度和加压气体二氧化碳作为来源,研究人员首次探索了将低碳酸氢盐浓度的废水溶液中的二氧化碳电还原成增值化合物。本研究探讨了在微流控条件下,从模拟和真实再生废水中,从酸化至pH 4 (15-50 mg-C L-1)后的低初始总无机碳(TIC0)浓度(指总无机碳(TIC))在压滤池中生产甲酸(FA)的可行性。结果表明,在japp = 5 mA cm-2时,最佳的TIC0为20 mg-C L-1,可平衡转化率(56%)、法拉第效率(FE)(9%)和比能耗(Esp) (1.96 kWh mol CO2-1)。将japp从5 mA cm-2减少到2 mA cm-2导致FE增加14%,尽管伴随着总体转换效率的降低,突出了选择性和生产率之间存在的妥协。基于TIC降解和FA生成的动力学模型准确地捕捉了实验趋势。实际出水结果表明,FA的生产是成功的。这些发现强调了质量输运(km)限制、TIC可用性和析氢反应(HER)等竞争反应之间的相互依存关系。km (1.4 ~ 1.6 × 10-5 m s-1)的变化表明,质量输移约束对CO2还原效率起关键作用,在较高的TIC0下,扩散层(δ)的增加限制了CO2在阴极表面的可用性,改变了CO2还原和HER之间的平衡。FTIR分析进一步显示,FA波段随着电流的增加而增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


