Astragaloside VI attenuates mechanical stress-induced cardiac remodeling through piezo1-VDAC1 dependent endoplasmic reticulum unfolded protein response
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
The dysregulation of protein homeostasis is a condition associated with mechanical stress-induced cardiac remodeling (CR) due to endoplasmic reticulum (ER) dysfunction and stress.
Purpose
This research explores the effect of Piezo1 on the ER unfolded protein response (UPR) in cardiomyocytes following hypoxic stress, specifically through its interaction with VDAC1. In addition, the study evaluates the therapeutic potential that this mechanism holds for treating CR and cardiomyocyte hypertrophy.
Study Design
Considering the relative limitation of potential therapeutic drugs for CR, our goal is to utilize a multi-omics approach to confirm the process by which Astragaloside IV (AS) alleviates CR through the Piezo1-VDAC1 dependent UPR.
Methods
We utilized multiple omics studies, such as single-cell sequencing, network pharmacology, and metagenomics, for the validation of AS's targets and phenotypic mechanisms. Following this, we created Piezo1/VDAC1 transgenic mice (Piezo1TG/VDAC1TG) and wild-type mice, which were then subjected to transverse aortic constriction (TAC) to induce myocardial damage. We performed assessments of cardiac function, myocardial injury staining, and cardiomyocyte hypertrophy on these animal models both before and after the drug intervention. The analysis into the interaction between Piezo1-VDAC1 and the structural integrity of cytoskeletal proteins and the ER was conducted utilizing laser confocal microscopy, immunofluorescence, and molecular biology experiments.
Results
The regulation of mechanical stress-induced cardiac remodeling crucially involves Piezo1-VDAC1. Data from single-cell sequencing and network pharmacology suggest that ER damage, mitochondrial energy metabolism dysfunction, and the dysregulation of subcellular organelles are important phenotypes that mediate this process. Our animal experiments demonstrated that AS is capable of improving cardiac function after TAC, inhibiting myocardial injury and the associated inflammatory reaction, and suppressing excessive UPR stress. The therapeutic effect of the drug was eliminated by the transgenic treatment of Piezo1. In vitro experiments also offered confirmation that AS can ameliorate cardiomyocyte damage through the ER pathway. This is achieved by regulating the Piezo1-VDAC1 interaction mechanism, which restores ER structural collapse after hypoxic injury, enhances energy metabolism levels, and inhibits excessive UPR stress.
Conclusion
The abnormal activation of the UPR, which is mediated by Piezo1-VDAC1, constitutes the pathological mechanism behind mechanical stress-induced cardiac remodeling. By regulating the Piezo1-VDAC1 interaction, AS inhibits excessive UPR stress and improves the breakdown of ER structure and functional abnormalities. These actions further normalize ER function and ameliorate cardiac function and myocarditis-related injury. This work offers a promising strategy for utilizing natural medicine to treat mechanical stress-induced cardiac remodeling.
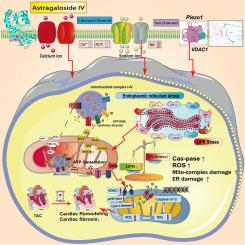
黄芪甲苷VI通过piezo1-VDAC1依赖性内质网未折叠蛋白反应减弱机械应力诱导的心脏重构。
背景:由于内质网(ER)功能障碍和应激,蛋白质稳态失调与机械应力诱导的心脏重构(CR)有关。目的:本研究探讨Piezo1对缺氧应激后心肌细胞内质网未折叠蛋白反应(UPR)的影响,特别是通过其与VDAC1的相互作用。此外,该研究还评估了该机制在治疗CR和心肌细胞肥大方面的治疗潜力。研究设计:考虑到CR的潜在治疗药物的相对局限性,我们的目标是利用多组学方法来确认黄芪甲苷(Astragaloside IV, AS)通过Piezo1-VDAC1依赖性UPR缓解CR的过程。方法:利用单细胞测序、网络药理学和宏基因组学等多组学研究,验证as的靶点和表型机制。在此基础上,我们建立了Piezo1/VDAC1转基因小鼠(Piezo1TG/VDAC1TG)和野生型小鼠,然后对它们进行横断主动脉收缩(TAC)诱导心肌损伤。我们在药物干预前后对这些动物模型进行了心功能、心肌损伤染色和心肌细胞肥大的评估。利用激光共聚焦显微镜、免疫荧光和分子生物学实验分析了Piezo1-VDAC1与细胞骨架蛋白和内质网结构完整性的相互作用。结果:机械应力诱导的心脏重构的调控主要涉及Piezo1-VDAC1。来自单细胞测序和网络药理学的数据表明,内质网损伤、线粒体能量代谢功能障碍和亚细胞器失调是介导这一过程的重要表型。我们的动物实验表明,AS能够改善TAC后的心功能,抑制心肌损伤及相关炎症反应,抑制过度的UPR应激。通过对Piezo1进行转基因处理,消除了药物的治疗效果。体外实验也证实AS可通过ER途径改善心肌细胞损伤。这是通过调节Piezo1-VDAC1相互作用机制来实现的,该机制可以恢复缺氧损伤后ER结构的崩溃,提高能量代谢水平,抑制过度的UPR应激。结论:由Piezo1-VDAC1介导的UPR异常激活是机械应力诱导心脏重构的病理机制。通过调节Piezo1-VDAC1相互作用,AS抑制了过度的UPR应力,改善了内质膜结构的破坏和功能异常。这些作用进一步使内质网功能正常化,改善心功能和心肌炎相关损伤。这项工作为利用天然药物治疗机械应力诱导的心脏重构提供了一个有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


