The role of dopaminergic signaling in insect response to repeated acute heat and cold stress
IF 2.9
2区 生物学
Q2 BIOLOGY
引用次数: 0
Abstract
As climate change accelerates, organisms face increasing exposure to unpredictable heat and cold extremes, threatening survival, reproduction, and long-term population stability. Understanding the physiological mechanisms underlying thermal resilience is essential to predict species responses and mitigate biodiversity loss. This study investigates the role of dopamine (DA) and its related receptors in mediating thermal tolerance and regulating life history traits in Drosophila melanogaster. Using wild-type Canton-S flies, we exposed adults to repeated acute thermal stress—daily 1-h cold (15 °C) or heat (35 °C) shocks for five to seven days—alongside a control group maintained at 25 °C. We assessed survival, fecundity, developmental time, adult emergence, and sex ratio. DA levels, measured by HPLC, significantly increased under heat stress and declined under cold exposure, suggesting its involvement in thermal response signaling. To further explore receptor-specific roles, we examined dopaminergic receptor mutants in Dop1R1, Dop1R2, Dop2R, DopEcR under the same thermal conditions described above. Our findings reveal that deficiencies in specific DA receptors markedly influenced survival and reproduction under thermal stress. For instance, Dop1R2 mutants exhibited high fecundity but poor survival under heat shock, while DopEcR mutants showed reduced fecundity under both cold and heat stress without affecting survival. Dop1R1 and Dop2R mutants exhibited developmental delays under thermal stress, indicating their contribution to life cycle regulation. These findings highlight dopaminergic signaling as a critical modulator of thermal adaptation, with implications for understanding how neurophysiological plasticity may buffer or limit insect resilience to future climate variability.
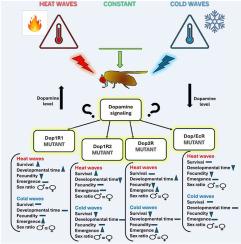
多巴胺能信号在昆虫对反复急性冷热应激反应中的作用。
随着气候变化的加速,生物面临越来越多的不可预测的极端高温和低温,威胁着生存、繁殖和长期人口稳定。了解热恢复的生理机制对预测物种反应和减轻生物多样性丧失至关重要。本研究探讨了多巴胺(DA)及其相关受体在黑腹果蝇(Drosophila melanogaster)热耐受性介导和生活史性状调控中的作用。使用野生型Canton-S蝇,我们将成虫暴露在重复的急性热应激中,每天1小时的冷(15°C)或热(35°C)冲击,持续5至7天,同时对照组保持在25°C。我们评估了存活率、繁殖力、发育时间、成虫羽化和性别比。高效液相色谱法测定的DA水平在热应激下显著升高,在冷暴露下显著下降,提示其参与热反应信号传导。为了进一步探索受体的特异性作用,我们在上述相同的热条件下检测了Dop1R1、Dop1R2、Dop2R和DopEcR中的多巴胺能受体突变体。我们的研究结果表明,特定DA受体的缺乏显著影响热应激下的生存和繁殖。例如,Dop1R2突变体在热休克下繁殖力高,但成活率差,而DopEcR突变体在冷应激和热应激下繁殖力均下降,但不影响成活率。Dop1R1和Dop2R突变体在热胁迫下表现出发育迟缓,表明它们对生命周期调控有贡献。这些发现强调了多巴胺能信号作为热适应的关键调节剂,对理解神经生理可塑性如何缓冲或限制昆虫对未来气候变化的适应能力具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of thermal biology
生物-动物学
CiteScore
5.30
自引率
7.40%
发文量
196
审稿时长
14.5 weeks
期刊介绍:
The Journal of Thermal Biology publishes articles that advance our knowledge on the ways and mechanisms through which temperature affects man and animals. This includes studies of their responses to these effects and on the ecological consequences. Directly relevant to this theme are:
• The mechanisms of thermal limitation, heat and cold injury, and the resistance of organisms to extremes of temperature
• The mechanisms involved in acclimation, acclimatization and evolutionary adaptation to temperature
• Mechanisms underlying the patterns of hibernation, torpor, dormancy, aestivation and diapause
• Effects of temperature on reproduction and development, growth, ageing and life-span
• Studies on modelling heat transfer between organisms and their environment
• The contributions of temperature to effects of climate change on animal species and man
• Studies of conservation biology and physiology related to temperature
• Behavioural and physiological regulation of body temperature including its pathophysiology and fever
• Medical applications of hypo- and hyperthermia
Article types:
• Original articles
• Review articles
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


