Polysaccharide extracted from Polygonatum kingianum Coll. Et Hemsl. Activates macrophages via the TLR4/NF-κB/MAPK pathway and exhibits vaccine adjuvant effect
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Polygonatum kingianum Coll. et Hemsl. (Huang Jing), a traditional Chinese medicinal herb, has long been used as a functional food and immune-enhancing remedy. Its polysaccharide component (PKP) is believed to drive immunomodulatory effects, though the molecular mechanisms behind its adjuvant potential remain unclear. PKP was isolated using water extraction, ethanol precipitation, enzymatic digestion, and purification. The immunostimulatory activity was assessed in vitro using RAW264.7 macrophages and primary macrophages from TLR4+/+ and TLR4−/− mice. Cytokine secretion (TNF-α, IL-6), nitric oxide production, and gene/protein expression were evaluated through RT-PCR, Western blotting, and immunofluorescence to analyze the activation of the TLR4/NF-κB/MAPK pathway. In vivo, the adjuvant activity of PKP was tested in BALB/c mice immunized with ovalbumin (OVA), measuring antigen-specific immune responses. The results revealed that PKP robustly activated macrophages in a TLR4-dependent manner, significantly enhancing TNF-α (3.8-fold) and IL-6 (2.5-fold) secretion, and upregulating MyD88, TRIF, p65-NF-κB, and MAPK phosphorylation. TLR4−/− macrophages showed abolished responses, confirming TLR4 as the critical receptor. PKP also enhanced antigen-specific IgG titers and Th1/Th2 cytokine production (IFN-γ, IL-4) in OVA-immunized mice, demonstrating its adjuvant efficacy. Notably, while PKP exhibited activity at higher in-vitro concentrations than LPS, its low toxicity and plant origin constitute key advantages over bacterial endotoxins; accordingly, PKP should be framed not as a more potent LPS alternative, but as a safer adjuvant candidate for vaccine development. In conclusion, PKP activates macrophages through the TLR4/MyD88/TRIF/NF-κB/MAPK axis and functions as a potent vaccine adjuvant, providing mechanistic validation for its traditional use and positioning it as a promising low-toxicity candidate for improving vaccine-mediated immunity.
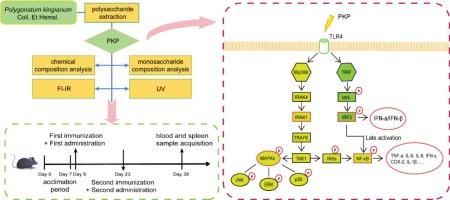
金黄精多糖的提取。Et Hemsl。通过TLR4/NF-κB/MAPK通路激活巨噬细胞,发挥疫苗佐剂作用。
黄精et Hemsl。黄菁是一种传统的中草药,长期以来一直被用作功能性食品和增强免疫力的药物。其多糖成分(PKP)被认为驱动免疫调节作用,尽管其辅助潜能背后的分子机制尚不清楚。采用水提、乙醇沉淀、酶解和纯化分离PKP。采用TLR4+/+和TLR4-/-小鼠RAW264.7巨噬细胞和原代巨噬细胞体外免疫刺激活性评估。通过RT-PCR、Western blotting和免疫荧光分析细胞因子分泌(TNF-α、IL-6)、一氧化氮生成和基因/蛋白表达,分析TLR4/NF-κB/MAPK通路的激活情况。在体内,用卵清蛋白(OVA)免疫BALB/c小鼠,检测PKP的佐剂活性,测定抗原特异性免疫反应。结果显示,PKP以tlr4依赖的方式强烈激活巨噬细胞,显著增强TNF-α(3.8倍)和IL-6(2.5倍)的分泌,上调MyD88、TRIF、p65-NF-κB和MAPK的磷酸化。TLR4-/-巨噬细胞表现出抑制反应,证实TLR4是关键受体。PKP还提高了ova免疫小鼠的抗原特异性IgG滴度和Th1/Th2细胞因子(IFN-γ, IL-4)的产生,证明了其佐剂作用。值得注意的是,尽管PKP在体外浓度高于LPS时表现出活性,但其低毒性和植物来源是其优于细菌内毒素的关键优势;因此,PKP不应被视为更有效的LPS替代品,而应被视为更安全的疫苗开发佐剂候选物。总之,PKP通过TLR4/MyD88/TRIF/NF-κB/MAPK轴激活巨噬细胞,作为一种有效的疫苗佐剂,为其传统用途提供了机制验证,并将其定位为一种有前途的低毒性候选物,可改善疫苗介导的免疫。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


