Molecular basis of the biogenesis of a protein organelle for ethanolamine utilization
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
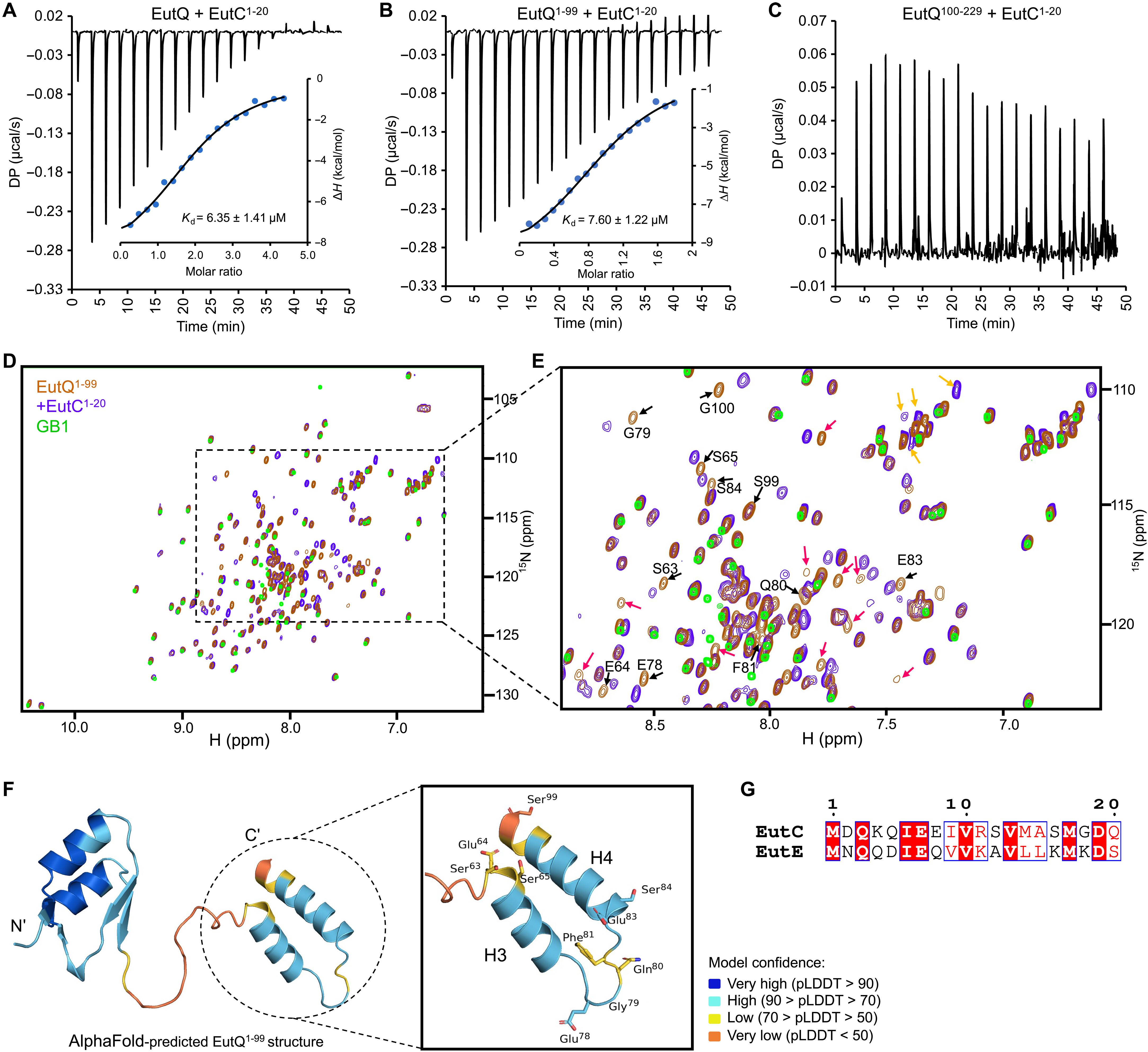
Many pathogenic bacteria use proteinaceous ethanolamine utilization microcompartments (Eut BMCs) to catabolize ethanolamine. This ability gives pathogens a competitive edge over commensal microbiota, which can drive virulence in the inflamed gut. Despite such a critical function, the molecular mechanisms underlying the synthesis of Eut BMCs remain elusive. We report a systematic study for dissecting the molecular basis underlying Eut BMC assembly in Salmonella. We determined the functions of individual constituent proteins in the structure and function of Eut BMCs and demonstrated that EutQ is essential for cargo encapsulation and Eut BMC formation through specific association with the shell and cargo enzymes. We found that Eut proteins can self-assemble to form cargo and shell aggregates independently in vivo and that the biogenesis of Eut BMCs follows a “shell-initiated” pathway. Cargo enzymes exhibit dynamic liquid-like organization within the Eut BMC. Our findings provide mechanistic insights into the structure and assembly of the Eut BMC that serves as a paradigm for membraneless organelles.
利用乙醇胺的蛋白质细胞器生物发生的分子基础
许多致病菌利用蛋白乙醇胺利用微室(BMCs)来分解乙醇胺。这种能力使病原体比共生微生物群具有竞争优势,共生微生物群可以在发炎的肠道中驱动毒性。尽管具有如此重要的功能,但Eut bmc合成的分子机制仍然难以捉摸。我们报告了一项系统的研究,解剖了沙门氏菌中Eut BMC组装的分子基础。我们确定了单个组成蛋白在Eut BMC结构和功能中的功能,并证明EutQ通过与壳酶和货酶的特异性结合,对货物封装和Eut BMC形成至关重要。我们发现,Eut蛋白可以在体内独立自组装形成货物和壳聚集体,并且Eut bmc的生物发生遵循“壳启动”途径。货物酶表现出动态的液体状组织在Eut BMC内。我们的发现为Eut BMC的结构和组装提供了机制见解,作为无膜细胞器的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


