Multi-level high hydrophilicity nanostructure electrocatalyst to promote H2 removal for efficient hydrogen evolution reaction
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
For large-scale utilization of renewable energy, the production of green hydrogen as energy carrier is efficacious through water electrolysis. The development of low-cost electrocatalysts for the hydrogen evolution reaction (HER) is critical. Herein, the control synthesis of NF-Co3O4-Ni(OH)2-Co(OH)2 with multi-level nanostructure was proposed by the multi-step hydrothermal-electrodeposition strategy. With rational multi-layer structure design, the catalyst based on non-precious metals had larger specific surface area and exposure of more active sites, giving efficient HER catalytic activity. Upon optimization of synthesis process, the electrocatalyst displayed a Tafel slope of 84 mV·dec−1 and overpotential of 42 mV at a current density of 10 mA·cm−2 in 1.0 mol·L−1 KOH solution, which was only slightly lower than that of commercial Pt/C@NF catalyst (corresponding value of 30 mV and 54 mV·dec−1). In a 40-hour stability test, the fabricated catalyst was electrochemically stable, showing negligible performance degradation. Moreover, with the multi-level nanostructure, the electrode exhibited high hydrophilicity and aerophobia properties. This work has demonstrated that the rational design of multi-level nanostructures is an effective strategy to synthesize high-performance HER electrocatalyst with non-precious materials.
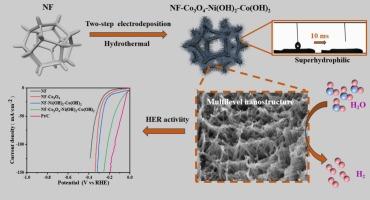
多级高亲水性纳米结构电催化剂促进H2脱除,实现高效析氢反应
对于可再生能源的大规模利用,通过水电解生产绿色氢作为能源载体是有效的。开发低成本的析氢反应电催化剂至关重要。本文提出了多级纳米结构的NF-Co3O4-Ni(OH)2- co (OH)2的控制合成方法。通过合理的多层结构设计,非贵金属催化剂具有更大的比表面积和更多的活性位点,具有高效的HER催化活性。在1.0 mol·L−1 KOH溶液中,当电流密度为10 mA·cm−2时,电催化剂的Tafel斜率为84 mV·dec−1,过电位为42 mV,仅略低于商品Pt/C@NF催化剂的相应值30 mV和54 mV·dec−1。在40小时的稳定性测试中,制备的催化剂是电化学稳定的,性能下降可以忽略不计。此外,由于多层纳米结构,电极具有较高的亲水性和厌氧性。研究表明,合理设计多层纳米结构是利用非贵重材料合成高性能HER电催化剂的有效策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


