Modular train-style nanorobots for targeted deep penetration and multi-directional collaborative treatment of colorectal cancer
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Colorectal cancer (CRC) remains a major global health challenge due to insufficient tumor penetration and immunosuppressive microenvironments. Herein, we propose a modular train-style nanorobot (TPP-Exo@LOX-Pd-Cu7S4) as a targeted synergistic therapeutic platform for CRC. The exosome “head” enables neutrophil-like tumor homing, while the Cu7S4 “tail” generates thermophoretic propulsion for deep tumor penetration. Under near-infrared region II (NIR-II) laser irradiation, the Pd-Cu7S4 Schottky heterojunction drives highly efficient catalytic cascades, disrupting redox homeostasis and inducing metabolic stress by converting O₂ to ·O₂−, H₂O₂ to ·OH, GSH to GSSG, NADH to NAD+, and lactate to pyruvate. The nanorobot directly targets mitochondria to reprogram tumor metabolism and trigger cuproptosis. Meanwhile, lactate oxidase (LOX), encapsulated within the engineered exosomes, depletes excess lactate to relieve immunosuppression and boost antitumor immunity. In CRC models, these nanorobots exhibit strong barrier penetration, precise targeting, and deep tumor infiltration, offering a multifunctional and metabolically disruptive therapeutic approach.
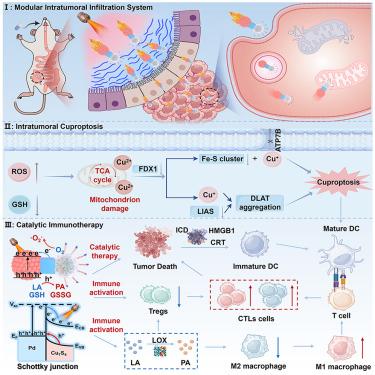
用于大肠癌靶向深度穿透和多方向协同治疗的模块化列车式纳米机器人
由于肿瘤渗透不足和免疫抑制微环境,结直肠癌(CRC)仍然是一个主要的全球健康挑战。在此,我们提出了一个模块化列车式纳米机器人(TPP-Exo@LOX-Pd-Cu7S4)作为CRC的靶向协同治疗平台。外泌体“头部”使嗜中性粒细胞样肿瘤归巢,而Cu7S4“尾部”产生热电泳推进,使肿瘤深入穿透。在近红外II区(NIR-II)激光照射下,Pd-Cu7S4 Schottky异质结驱动高效催化级联反应,通过将O₂转化为·O₂−、H₂O₂转化为·OH、GSH转化为GSSG、NADH转化为NAD+、乳酸转化为丙酮酸,破坏氧化还原稳态并诱导代谢应激。该纳米机器人直接靶向线粒体来重编程肿瘤代谢并触发cuprotosis。同时,包裹在工程外泌体内的乳酸氧化酶(LOX)消耗多余的乳酸,以减轻免疫抑制和增强抗肿瘤免疫。在结直肠癌模型中,这些纳米机器人表现出强大的屏障穿透性、精确的靶向性和深度肿瘤浸润性,提供了一种多功能和代谢破坏性的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


