In situ enrichment and delivery of STING agonists by protamine-modified Salmonella for cancer immunotherapy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Immunotherapy has emerged as a promising therapeutic strategy for cancer. The activation of the stimulator of interferon genes (STING) pathway promotes the polarization of tumor-associated macrophages (TAMs) towards M1 phenotype, with 2′, 3′-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) serving as an inherent activator, which significantly accumulates in the extracellular space of the tumor sites following radiotherapy (RT). However, the electronegativity and hydrophilicity of cGAMP prevent it from crossing the cell membrane into TAMs, hampering subsequent immunotherapy efficacy. Here, positively charged protamine-modified Salmonella (VNP20009), called VNP-protamine (VNP-PRM), were prepared to enrich cGAMP and form a composite bacteria-drug delivery system with the capacity to enter TAMs freely. Via electrostatic interactions between the guanidine groups of protamine and the phosphate groups of cGAMP, VNP-PRM stably enriched cGAMP on their surface and neutralized the electronegativity of cGAMP. The uptake efficiency of cGAMP by TAMs was then significantly enhanced by the active delivery of VNP-PRM, whose inherent motility and cellular invasiveness endowed them with increased potential to bump against and enter the macrophages. Subsequently, the intracellular cGAMP synergized with the immunogenicity of the bacteria to activate the STING pathway and drive M1 polarization, thereby boosting tumor eradication. Therefore, antitumor immunotherapy can be optimized through in situ enrichment and delivery of post-RT cGAMP to TAMs by the engineered bacteria.
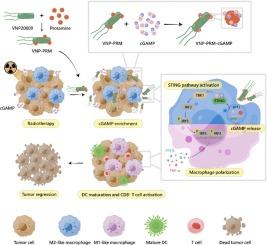

蛋白蛋白修饰的沙门氏菌原位富集和递送STING激动剂用于癌症免疫治疗
免疫疗法已成为一种很有前途的癌症治疗策略。干扰素基因刺激因子(STING)通路的激活促进肿瘤相关巨噬细胞(tam)向M1表型极化,其中2 ',3 ' -环鸟苷单磷酸-腺苷单磷酸(cGAMP)作为固有激活因子,在放疗(RT)后肿瘤部位的细胞外空间显著积累。然而,cGAMP的电负性和亲水性阻止了它穿过细胞膜进入tam,从而阻碍了随后的免疫治疗效果。本研究制备了带正电荷的蛋白蛋白修饰沙门氏菌(VNP20009),称为vnp -鱼精蛋白(VNP-PRM),以富集cGAMP,形成能够自由进入tam的复合细菌-药物传递系统。VNP-PRM通过鱼精蛋白的胍基与cGAMP的磷酸基之间的静电相互作用,稳定地在其表面富集cGAMP,并中和cGAMP的电负性。通过主动递送VNP-PRM, tam对cGAMP的摄取效率显著提高,其固有的运动性和细胞侵袭性使其具有更大的碰撞和进入巨噬细胞的潜力。随后,细胞内的cGAMP与细菌的免疫原性协同激活STING通路,驱动M1极化,从而促进肿瘤的根除。因此,可以通过工程细菌原位富集和递送rt后的cGAMP到tam中来优化抗肿瘤免疫治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


