Glioblastoma and blood-brain barrier cell interplay in vitro models for stratification of drug efficacy
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
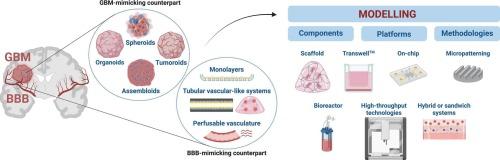
Glioblastoma (GBM) is the most lethal brain cancer in adults, with a dismal prognosis and no curative therapies available. The treatment landscape remains largely stagnant, relying on tumor resection, temozolomide (TMZ) chemotherapy, and radiotherapy, which are hampered by the blood-brain barrier (BBB) that limits drug blood-to-brain permeability and, consequently, therapeutic efficacy. Over 98 % of potential therapeutic candidates fail to penetrate the BBB, significantly contributing to the high recurrence rates of GBM. The urgent need for improved drug delivery strategies is compounded by the limitations of current preclinical models, which often inadequately mimic the complex BBB-GBM interaction. This review discusses recent advancements in the development of in vitro models that accurately replicate the BBB and GBM interplay, ranging from simplified two-dimensional (2D) systems to sophisticated three-dimensional (3D) constructs. Innovations such as microfluidic devices and multicellular spheroid cultures are highlighted as promising methods to enhance physiological relevance and predictive value in drug testing. By emphasizing the interplay between GBM and its microenvironment with the BBB, these models aim to accelerate the discovery and efficacy testing of novel anti-GBM agents. Ultimately, this review underscores the critical need for more representative in vitro platforms that not only reduce reliance on animal models but also adhere to the principles of the 3Rs (replacement, reduction, refinement) in biomedical research, paving the way for more effective therapeutic interventions against GBM.
胶质母细胞瘤和血脑屏障细胞相互作用的体外模型对药物疗效的分层
胶质母细胞瘤(GBM)是成人中最致命的脑癌,预后不佳,没有治愈的治疗方法。目前的治疗方案仍然停滞不前,主要依靠肿瘤切除、替莫唑胺(TMZ)化疗和放射治疗,但这些方法受到血脑屏障(BBB)的阻碍,血脑屏障限制了药物的血脑通透性,从而影响了治疗效果。超过98% %的潜在治疗候选物不能穿透血脑屏障,这是导致GBM高复发率的重要原因。由于目前临床前模型的局限性,迫切需要改进给药策略,这些模型往往不能充分模拟复杂的BBB-GBM相互作用。本文讨论了精确复制血脑屏障和GBM相互作用的体外模型的最新进展,范围从简化的二维(2D)系统到复杂的三维(3D)结构。微流体装置和多细胞球体培养等创新被强调为有希望的方法,以增强药物测试中的生理相关性和预测价值。通过强调GBM及其微环境与血脑屏障之间的相互作用,这些模型旨在加速新型抗GBM药物的发现和疗效测试。最后,这篇综述强调了对更具代表性的体外平台的迫切需要,不仅要减少对动物模型的依赖,而且要坚持生物医学研究中的3r(替代、减少、改进)原则,为更有效地治疗GBM铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


