Human umbilical cord blood cells-secreted exosomal MFG-E8 regulates microglia polarization and ameliorates hypoxic-ischemic brain damage in neonatal rats by SOCS3/STAT3 axis
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Objective
Stem cell therapy is expected to become a new treatment for central nervous system damage associated with perinatal hypoxic-ischemic encephalopathy (HIE), but the specific effects are unknown. This study explores the effects of human umbilical cord blood (HUCB) cells-secreted exosomal (HUCB-ex) MFG-E8 in neonatal rats with hypoxic-ischemic brain damage (HIBD), aiming to gain a theoretical foundation for the cure of perinatal HIE.
Methods
HIBD model was constructed in the Sprague Dawley rats (7-day-old). Rats were then intervened with 1 × 106 HUCB cells, HUCB-ex, or HUCB-exoe-MFG-E8, and HUCB-exsi-MFG-E8. Primary microglia from rats were induced with oxygen-glucose deprivation and re-oxygenation (OGD/R), then co-cultured with either HUCB or primary neuronal cells, and subjected to treatment with HUCB-ex, HUCB-exoe-MFG-E8, HUCB-exsi-MFG-E8, or Stattic. The expression of polarization factors and secreted factors in the microglia was measured using RT-qPCR, immunofluorescence, and Western blot. Neuronal cell damage was assessed using MTT assays and flow cytometry. Behavioral impairments and brain tissue damage in the rats were evaluated using assays including the geotaxis reflex, cliff avoidance response, grip strength test, hematoxylin-eosin staining, TTC staining, and immunofluorescence.
Results
Early intervention with HUCB cells in HIBD rats increased test scores, decreased brain tissue weight, infarct area, as well as the IL-6, TNF-α, and IL-1β levels, and increased MFG-E8 levels. HUCB cells also decreased the levels of CD11b/c+CD45hi cells in HIBD rat brain tissue, and increased the levels of CD206+CD11b/c+ cells. In vitro experiments confirmed high expression of MFG-E8 in HUCB-ex. HUCB-exsi-MFG-E8 inhibited M2 polarization and induced neuronal cell injury through the SOCS3/STAT3 pathway. HUCB-ex and HUCB cells have equivalent therapeutic effects in HIBD rats. The treatment effectiveness of HUCB-ex was improved after delivering HUCB-exoe-MFG-E8, while was blocked after delivering HUCB-exsi-MFG-E8.
Conclusions
HUCB-exoe-MFG-E8 promoted M2 polarization of microglial and inhibited neuronal cell apoptosis through the SOCS3/STAT3 pathway, to alleviate behavioral disorders and brain tissue damage in HIBD rats.
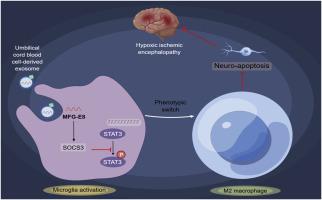
人脐带血细胞分泌外泌体MFG-E8通过SOCS3/STAT3轴调控小胶质细胞极化,改善新生大鼠缺氧缺血性脑损伤。
目的:干细胞治疗有望成为围产期缺氧缺血性脑病(HIE)相关中枢神经系统损伤的一种新的治疗方法,但具体效果尚不清楚。本研究探讨人脐带血(HUCB)细胞分泌外泌体(HUCB-ex) MFG-E8在新生大鼠缺氧缺血性脑损伤(HIBD)中的作用,旨在为围产期HIE的治疗提供理论依据。方法:建立7日龄大鼠HIBD模型。然后用1×106 HUCB细胞、HUCB-ex或HUCB-exoe- mfg - e8和HUCB- exi - mfg - e8干预大鼠。用氧-葡萄糖剥夺和再氧合(OGD/R)诱导大鼠原代小胶质细胞,然后与HUCB或原代神经元细胞共培养,分别用HUCB-ex、HUCB-exoe- mfg - e8、HUCB- exi - mfg - e8或Stattic处理。采用RT-qPCR、免疫荧光和western blot检测极化因子和分泌因子在小胶质细胞中的表达。采用MTT和流式细胞术评估神经元细胞损伤。采用地向反射、悬崖躲避反应、握力测试、苏木精-伊红染色、TTC染色、免疫荧光等方法评价大鼠的行为障碍和脑组织损伤情况。结果:早期干预hub细胞可提高HIBD大鼠的测试分数,降低脑组织重量、梗死面积,降低IL-6、TNF-α、IL-1β水平,升高MFG-E8水平。HUCB细胞还降低了HIBD大鼠脑组织中CD11b/c+CD45hi细胞的水平,增加了CD206+CD11b/c+细胞的水平。体外实验证实MFG-E8在HUCB-ex中高表达。HUCB-exsi-MFG-E8通过SOCS3/STAT3通路抑制M2极化,诱导神经元细胞损伤。HUCB-ex和HUCB细胞对HIBD大鼠具有相当的治疗作用。给药hub - exe - mfg - e8后,hub -ex的治疗效果得到提高,而给药hub - exi - mfg - e8后,hub -ex的治疗效果被阻断。结论:HUCB-exoe-MFG-E8通过SOCS3/STAT3通路促进小胶质细胞M2极化,抑制神经元细胞凋亡,减轻HIBD大鼠行为障碍和脑组织损伤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


