Deoxynivalenol toxicity along the gut–liver–brain axis in animal models: Mechanisms of action and strategies for mitigation
IF 3.5
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Deoxynivalenol (DON), a trichothecene mycotoxin commonly found in cereals and animal feed, exerts systemic toxicity affecting intestinal, hepatic, and neural function. This review critically examines DON-induced damage along the gut–liver–brain axis in animal models, highlighting shared molecular mechanisms including oxidative stress, inflammatory signaling, and impaired antioxidant responses. DON disrupts intestinal barrier integrity, alters microbiota composition, promotes hepatic inflammation and apoptosis, and can impair neurochemical balance via direct and gut-mediated pathways. We also summarize the prevalence of DON contamination globally, with detection rates exceeding 60 % in some regions. In response to these risks, various mitigation strategies have been explored. This review evaluates the efficacy and safety of bioactive compounds such as vitamins, plant polyphenols, probiotics, and yeast derivatives, as well as synthetic agents, in reducing DON toxicity. Mechanisms of action include antioxidant defense, mycotoxin binding, and modulation of host immunity. Despite progress, knowledge gaps remain in long-term exposure effects, combinatory mycotoxin toxicity, and feed additive standardization. Integrative and translational approaches are needed to ensure effective DON risk management in food-producing systems.
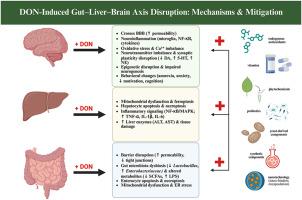
脱氧雪腐烯醇在动物模型中沿肠-肝-脑轴的毒性:作用机制和缓解策略。
脱氧雪腐镰刀菌醇(DON)是一种常见于谷物和动物饲料中的霉菌毒素,具有影响肠道、肝脏和神经功能的全身毒性。本综述在动物模型中严格检查了don诱导的肠-肝-脑轴损伤,强调了包括氧化应激、炎症信号和抗氧化反应受损在内的共同分子机制。DON破坏肠道屏障完整性,改变微生物群组成,促进肝脏炎症和凋亡,并可通过直接和肠道介导的途径损害神经化学平衡。我们还总结了全球DON污染的流行情况,一些地区的检出率超过60%。针对这些风险,探索了各种缓解战略。本文综述了生物活性化合物如维生素、植物多酚、益生菌、酵母衍生物以及合成制剂在降低DON毒性方面的有效性和安全性。其作用机制包括抗氧化防御、真菌毒素结合和宿主免疫调节。尽管取得了进展,但在长期接触效应、联合霉菌毒素毒性和饲料添加剂标准化方面仍存在知识空白。需要综合和转化的方法来确保粮食生产系统中DON风险的有效管理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


