Biologically relevant morpholino nucleoside thio- and dithiophosphates via an oxathiaphospholane approach
IF 2.5
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Appropriately protected morpholino nucleoside 6′-O-(2-thio)-1,3,2-oxathiaphospholanes and 6′-O-(2-thio)-1,3,2-dithiaphospholanes react with 3-hydroxypropionitrile in the presence of a strong base catalyst (DBU), yielding morpholino nucleoside 6′-O-(α-thiophosphates) and 6′-O-(α,α-dithiophosphates), respectively. The synthesized compounds exhibit low cytotoxicity toward human cells, indicating their favorable biocompatibility and potential for further biological and therapeutic applications.
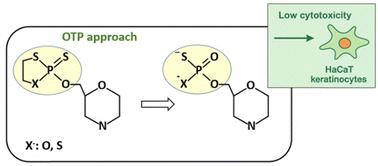
生物学上相关的硫代和二硫代磷酸盐通过草酸磷烷方法
适当保护的6′- o -(2-硫)-1,3,2-草硫代磷烷和6′- o -(2-硫)-1,3,2-二硫代磷烷在强碱催化剂(DBU)存在下与3-羟基丙腈反应,分别生成6′- o -(α-硫代磷酸盐)和6′- o -(α,α-二硫代磷酸盐)。合成的化合物对人体细胞具有较低的细胞毒性,表明其具有良好的生物相容性和进一步的生物和治疗应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

New Journal of Chemistry
化学-化学综合
CiteScore
5.30
自引率
6.10%
发文量
1832
审稿时长
2 months
期刊介绍:
A journal for new directions in chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


