Immunometabolic reprogramming of macrophages by gut microbiota-derived cadaverine controls colon inflammation
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Cadaverine is a polyamine produced by the gut microbiota with links to health and disease, notably inflammatory bowel disease (IBD). Here, we show that cadaverine shapes monocyte-macrophage immunometabolism in a context- and concentration-dependent fashion to impact macrophage functionality. At baseline, cadaverine is taken up via L-lysine transporters and activates the thioredoxin system, while during inflammation, cadaverine signals through aconitate decarboxylase 1 (Acod1)-itaconate. Both pathways induce activation of transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2), which supports mitochondrial respiration and promotes immunoregulatory macrophage polarization. Conversely, under higher concentrations, cadaverine acts via histamine 4 receptor, leading to glycolysis-driven inflammation and pro-inflammatory functions in macrophages. Likewise, cadaverine exhibits paradoxical effects in experimental colitis, either protective or detrimental, evoking opposite fates on macrophages depending on levels dictated by Enterobacteriaceae. In IBD patients, elevated cadaverine correlated with higher flare risk. Our findings implicate cadaverine as a microbiota-derived metabolite manipulating macrophage energy metabolism with consequences in intestinal inflammation and implications for IBD pathogenesis.
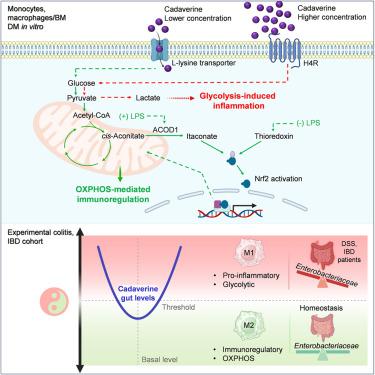
肠道微生物源性尸胺对巨噬细胞的免疫代谢重编程控制结肠炎症
尸胺是一种由肠道菌群产生的多胺,与健康和疾病有关,尤其是炎症性肠病(IBD)。在这里,我们表明尸胺以环境和浓度依赖的方式影响单核细胞-巨噬细胞的免疫代谢,从而影响巨噬细胞的功能。在基线时,尸胺通过l -赖氨酸转运体被吸收并激活硫氧还蛋白系统,而在炎症期间,尸胺通过aconitate decarboxylase 1 (Acod1)- itacon酸发出信号。这两种途径都诱导转录因子、核因子红细胞2相关因子2 (Nrf2)的激活,支持线粒体呼吸,促进免疫调节性巨噬细胞极化。相反,在较高浓度下,尸胺通过组胺4受体起作用,导致巨噬细胞糖酵解驱动的炎症和促炎功能。同样,尸胺在实验性结肠炎中表现出矛盾的作用,要么是保护性的,要么是有害的,根据肠杆菌科决定的水平,对巨噬细胞产生相反的命运。在IBD患者中,尸胺升高与更高的发作风险相关。我们的研究结果表明尸胺作为一种微生物衍生的代谢物,操纵巨噬细胞的能量代谢,影响肠道炎症和IBD的发病机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


