Additively manufactured microplate for the simultaneous colorimetric and electrochemical detection of atropine
IF 3.3
3区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
This work presents the development of a dual mode electrochemical and colorimetric sensing platform, produced in a single print through additive manufacturing. The cell design was based on the dimensions of a standard 96-well plate, with the base replaced by a disc electrode made from bespoke conductive polypropylene for the electrochemical testing, with the walls created from transparent non-conductive polypropylene to allow for the colorimetric tests. This new system was employed for the detection of atropine (ATP) in two distinct steps within the same electrochemical cell: (1) colour changes due to the reaction of ATP with bromocresol green, allowing for preliminary visual identification, and (2) the analysis of the electrochemical behaviour of the system before and after the colour change, providing quantitative confirmation. Both steps were performed in the same cell, highlighting the efficiency and practicality of the developed device. Wide linear ranges were obtained using square-wave voltammetry for ATP detection, spanning 0.65 to 20.83 mg mL−1 before the colorimetric reaction, and 5.21 to 20.83 mg mL−1 after the colorimetric reaction. Detection and quantification limits were calculated as 0.15 mg mL−1 and 0.50 mg mL−1, respectively, demonstrating suitability for real application in forensic scenarios. Beverage samples (energy drink, tonic water, gin, gin with tonic water, and whisky) and synthetic biological samples (saliva, urine, and vitreous humour) were spiked with ATP and analysed using the proposed method, yielding recoveries close to 100%, indicating no matrix effect. This study demonstrates the synergy between additive manufacturing, and electrochemical and colorimetric sensing to create real, functional sensing platforms that are applicable to a wide range of fields.
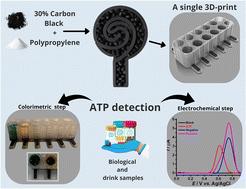
用于同时比色和电化学检测阿托品的增材制造微孔板
这项工作提出了一种双模式电化学和比色传感平台的发展,通过增材制造在单次打印中生产。电池的设计基于标准96孔板的尺寸,底座由定制的导电聚丙烯制成的圆盘电极代替,用于电化学测试,壁由透明的不导电聚丙烯制成,以便进行比色测试。该新系统用于在同一电化学电池内通过两个不同的步骤检测阿托品(ATP):(1)由于ATP与溴甲酚绿反应而引起的颜色变化,允许初步的视觉识别;(2)分析颜色变化前后系统的电化学行为,提供定量确认。这两个步骤都是在同一个电池中进行的,突出了所开发设备的效率和实用性。方波伏安法检测ATP的线性范围较宽,比色反应前为0.65 ~ 20.83 mg mL−1,比色反应后为5.21 ~ 20.83 mg mL−1。检测限和定量限分别计算为0.15 mg mL - 1和0.50 mg mL - 1,证明了在法医场景中的实际应用适用性。饮料样品(能量饮料、奎宁水、杜松子酒、杜松子酒加奎宁水和威士忌)和合成生物样品(唾液、尿液和玻璃体体液)加入ATP并使用所提出的方法进行分析,回收率接近100%,表明没有基质效应。这项研究展示了增材制造、电化学和比色传感之间的协同作用,以创建适用于广泛领域的真实、功能性传感平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analyst
化学-分析化学
CiteScore
7.80
自引率
4.80%
发文量
636
审稿时长
1.9 months
期刊介绍:
"Analyst" journal is the home of premier fundamental discoveries, inventions and applications in the analytical and bioanalytical sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


