Hydrogen Bonding at MXene/Electrolyte Interface Enables Stable Ammonium Ion Energy Storage
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Aqueous ammonium ion (NH4+) hybrid supercapacitors (AAHSCs) have attracted much attention due to their environmental friendliness and excellent electrochemical performance, but the mechanism of NH4+ energy storage of AAHSCs has been unknown. In this work, Ti3C2Tx films were prepared by vacuum filtration and used as cathode materials for AAHSC. The charge storage mechanism of Ti3C2Tx films in different electrolyte solutions was systematically analyzed by means of ex-situ techniques and theoretical calculations. These analyses revealed an H–bonding (N–H…O) interaction between NH4+ and oxygen-containing functional groups, elucidating the reason for the excellent electrochemical performance of AAHSCs. DFT and MD calculations further verified the existence of H–bonds, which act as a channel for charge transfer and facilitate the transfer of electrons from NH4+ to O–atoms. The Ti3C2Tx film//AC-AAHSC assembled with 1 M (NH4)2SO4 as the electrolyte solution has a high specific capacitance of 84.3 F/g at a current density of 1 A/g and excellent cycling stability of 96.2% (10,000 cycles). It has a high energy density of 108 Wh/kg at a power density of 2,160 W/kg. This work lays the theoretical foundation for the construction of high-performance MXene-based AAHSCs devices.
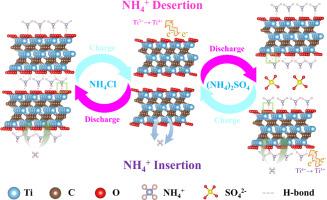
MXene/电解质界面氢键实现稳定的铵离子储能
水铵离子(NH4+)混合超级电容器(AAHSCs)因其环境友好性和优异的电化学性能而备受关注,但其NH4+储能机理尚不清楚。本文采用真空过滤法制备了Ti3C2Tx薄膜,并将其作为AAHSC的正极材料。采用非原位技术和理论计算的方法,系统分析了Ti3C2Tx薄膜在不同电解质溶液中的电荷存储机理。这些分析揭示了NH4+与含氧官能团之间的氢键(N-H…O)相互作用,阐明了AAHSCs具有优异电化学性能的原因。DFT和MD计算进一步验证了氢键的存在,氢键作为电荷转移的通道,促进了电子从NH4+向o原子的转移。以1 M (NH4)2SO4为电解液组装的Ti3C2Tx薄膜//AC-AAHSC在1 a /g电流密度下具有84.3 F/g的高比电容和96.2%的优良循环稳定性(10,000次循环)。功率密度为2160 W/kg,能量密度为108 Wh/kg。本工作为构建基于mxene的高性能AAHSCs器件奠定了理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


