Enhanced solubility of carbamazepine using cholinium-based ionic liquid: From COSMO-RS screening to molecular dynamics simulation
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
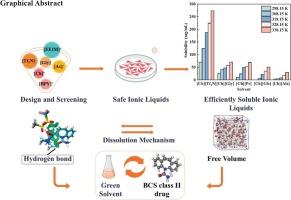
In this study, the objective was to identify a suitable cholinium-based ionic liquid (IL) for carbamazepine (CBZ), a Biopharmaceutics Classification System (BCS) Class II drug. The solubility and dissolution mechanism of CBZ in various ILs were systematically investigated. Potential ILs were screened using the logarithm of the infinite-dilution activity coefficient (lnγ∞) derived from the Conductor-like Screening Model for Real Solvents (COSMO-RS) model. Through the integration of experimental results and COSMO-RS model predictions, CBZ was found to exhibit the highest solubility (70.37 mg/mL) in cholinium bis(trifluoromethylsulfonyl)imide ([Ch][Tf2N]) at 25 °C, a 600-fold increase over its solubility in water. Moreover, cell assays confirmed the low-toxic nature of [Ch][Tf2N]. Quantum chemical calculations and molecular dynamics simulations revealed that hydrogen bonds formed by electrostatic interactions between [Ch][Tf2N] and CBZ, together with the significant free volume provided by [Ch][Tf2N], synergistically enhance CBZ dissolution by stabilizing drug-solvent interactions and facilitating molecular mobility. Cholinium-based ILs, characterized by low toxicity and remarkable dissolution efficiency, can serve as cosolvents in novel formulations for poorly soluble drugs, demonstrating substantial development potential. This study offers valuable insights into enhancing the dissolution of BCS Class II poorly soluble drugs with similar structural features.
基于胆碱的离子液体增强卡马西平的溶解度:从cosmos - rs筛选到分子动力学模拟
本研究的目的是为生物制药分类系统(BCS)第二类药物卡马西平(CBZ)寻找合适的胆碱离子液体(IL)。系统地研究了CBZ在各种il中的溶解度和溶解机理。利用基于类导体筛选模型(cosmos - rs)模型的无限稀释活度系数(lnγ∞)的对数来筛选潜在的ILs。通过综合实验结果和cosmos - rs模型预测,发现CBZ在25 ℃时在双(三氟甲基磺酰基)酰胆碱亚胺([Ch][Tf2N])中具有最高的溶解度(70.37 mg/mL),比其在水中的溶解度提高了600倍。此外,细胞实验证实了[Ch][Tf2N]的低毒性质。量子化学计算和分子动力学模拟表明,[Ch][Tf2N]与CBZ之间静电相互作用形成的氢键,以及[Ch][Tf2N]提供的大量自由体积,通过稳定药物-溶剂相互作用和促进分子迁移,协同增强了CBZ的溶解。胆碱类化合物具有毒性低、溶出效率高的特点,可作为新型难溶性药物的共溶剂,具有很大的开发潜力。本研究为促进具有相似结构特征的BCS II类难溶性药物的溶出提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


