Selective separation of quercetin and p-Coumaric acid based on new Hansen solubility parameters
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Polyphenols such as quercetin and p-coumaric acid are valuable bioactive compounds, but their selective separation remains a challenge due to their structural similarity and coexistence in natural matrices. In this work, the selective separation of quercetin and p-coumaric acid is addressed based on experimental solubility studies and calculated new Hansen solubility parameters (HSPs). Solubility of both polyphenols was first determined in 21 mono-solvents at 298.15 K and 0.1 MPa and subsequently employed for HSP calculation. Theoretical HSP-based predictions were validated with solubility measurements in binary solvent mixtures combining methanol, ethanol, methyl ethyl ketone (MEK), and methyl isobutyl ketone (MiBK). Based on preliminary solubility results and calculated HSPs, selectivity of methanol and MiBK with quercetin and p-coumaric acid was evaluated. Three-dimensional Hansen spheres showed strong agreement with preliminary solubility data in mono-solvents. HSP-based predictions revealed solubility maxima in those mixtures composed of solvents with different functional groups, in accordance with experimental results. As for selectivity tests, MiBK showed high extraction efficiencies for both compounds, whereas methanol exhibited great potential in selectively separating p-coumaric acid. Eventually, a two-step separation procedure is proposed, using methanol first to selectively extract p-coumaric acid, and subsequently employing MiBK to purify residual quercetin. Overall, this work underscores the importance of experimental HSP-based approaches and Hansen theory in solubility prediction and solvent selection strategies for the separation of bioactive compounds from natural sources.
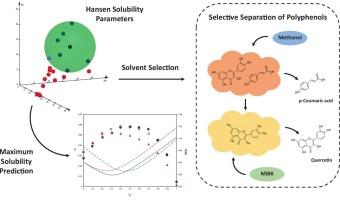
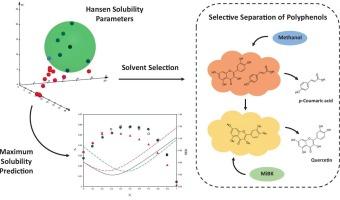
基于Hansen溶解度新参数的槲皮素和对香豆酸的选择性分离
槲皮素和对香豆酸等多酚类化合物是有价值的生物活性化合物,但由于它们在天然基质中的结构相似性和共存性,它们的选择性分离仍然是一个挑战。在本工作中,基于实验溶解度研究和计算新的汉森溶解度参数(HSPs),解决了槲皮素和对香豆酸的选择性分离问题。首先在298.15 K和0.1 MPa条件下测定两种多酚在21种单溶剂中的溶解度,然后进行热sp计算。通过测量甲醇、乙醇、甲基乙基酮(MEK)和甲基异丁基酮(MiBK)组成的二元溶剂混合物中的溶解度,验证了基于热热蛋白的理论预测。根据初步溶解度结果和计算的热敏感值,评价了甲醇和MiBK对槲皮素和对香豆酸的选择性。三维汉森球在单溶剂中的溶解度与初步数据一致。根据实验结果,基于热热蛋白的预测显示,在由具有不同官能团的溶剂组成的混合物中,溶解度最大。在选择性测试中,MiBK对这两种化合物的萃取效率都很高,而甲醇在对香豆酸的选择性分离中表现出很大的潜力。最后,我们提出了一种两步分离方法,首先用甲醇选择性地提取对香豆酸,然后用MiBK纯化残留的槲皮素。总的来说,这项工作强调了基于热蛋白的实验方法和汉森理论在从天然来源分离生物活性化合物的溶解度预测和溶剂选择策略中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


