A novel Halo Tag-based photo-crosslinking system for efficient drug target identification via chemoproteomic profiling
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
Target discovery of natural products is critical for novel drug development. However, it remains challenging due to the risk of disrupting bioactive molecule integrity during chemical modification. Moreover, many current methods for identifying drug targets rely on chemical synthesis to design the probes and enrichment systems. This is not friendly to researchers lacking experience in chemical synthesis, which limits their large-scale applications. To address these limitations, we present a novel chemoproteomic strategy to identify drug targets by integrating Halo Tag technology with photo-crosslinking chemistry.
Results
A bifunctional probe was synthesized by conjugating the Halo Tag ligand with a photo-crosslinker, 3-Phenyl-3-(trifluoromethyl)-3H-diazirine (TAD). TAD induced UV-triggered covalent binding with drug molecules. And Halo Tag ligand was enriched by Halo Protein magnetic beads, with less structural bias and reduced activity impairment. Taking sweroside (SWE) as an example, 159 target proteins were screened using this probe, and yin-yang 1 (YY1) was selected as the most potential target of SWE. Furtherly, in vitro small molecule-protein interaction analysis, including cell thermal shift assays (CETSA), surface plasmon resonance (SPR) and molecular docking were performed. These results demonstrated the strong binding affinity of YY1 and SWE. Moreover, SWE was demonstrated to relieve bile acid (BA) induced hepatocyte apoptosis by targeting YY1 to regulate its transcriptional activity to enhance Farnesoid X Receptor (FXR) expression. Furthermore, in vivo assays showed that SWE administration effectively ameliorated 3,5-diethoxycarbonyl-1,4-dihydro-collidine (DDC) induced cholestatic liver injury in mice. It was evidenced by markedly reducing the serum biochemical biomarkers and liver pathological change. Notably, SWE decreased YY1 expression and promoted FXR and BSEP expression. Thus, SWE exerted hepatoprotective effects by regulating YY1/FXR pathway.
Significance
In conclusion, our method without pre-derivatization simplifies workflows and preserves native pharmacophores. It is compatible with routine laboratory equipment and can be widely used in drug target discovery.
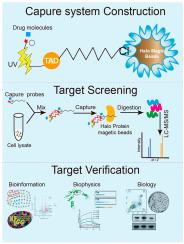
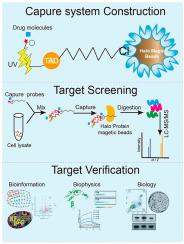
一种基于光晕标签的光交联系统,可通过化学蛋白质组学分析高效识别药物靶标
天然产物靶点的发现对新药开发至关重要。然而,由于化学修饰过程中破坏生物活性分子完整性的风险,这仍然具有挑战性。此外,目前许多识别药物靶点的方法依赖于化学合成来设计探针和富集系统。这对缺乏化学合成经验的研究人员来说是不友好的,这限制了他们的大规模应用。为了解决这些限制,我们提出了一种新的化学蛋白质组学策略,通过将光晕标签技术与光交联化学相结合来识别药物靶点。结果通过光交联剂3-苯基-3-(三氟甲基)- 3h -重氮嗪(TAD)与Halo标签配体偶联合成了双功能探针。TAD诱导紫外触发的与药物分子的共价结合。Halo蛋白磁珠富集了Halo标签配体,减少了结构偏倚,减少了活性损害。以雪薇苷(SWE)为例,利用该探针筛选了159个靶蛋白,其中阴阳1 (YY1)是SWE最有潜力的靶点。此外,还进行了体外小分子-蛋白相互作用分析,包括细胞热移分析(CETSA)、表面等离子体共振(SPR)和分子对接。这些结果表明YY1与SWE具有很强的结合亲和力。此外,SWE通过靶向YY1调控其转录活性,增强Farnesoid X受体(FXR)的表达,从而缓解胆汁酸(BA)诱导的肝细胞凋亡。此外,体内实验表明,SWE可有效改善3,5-二氧羰基-1,4-二氢碰撞碱(DDC)诱导的小鼠胆汁淤积性肝损伤。血清生化指标和肝脏病理改变均明显降低。值得注意的是,SWE降低了YY1的表达,促进了FXR和BSEP的表达。因此,SWE通过调节YY1/FXR通路发挥肝保护作用。总之,我们的方法无需预衍生化,简化了工作流程并保留了天然药效团。它与常规实验室设备兼容,可广泛用于药物靶点发现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


