Per- and polyfluoroalkyl substances (PFAS) disrupt gut microbiome composition and metabolism in metabolic syndrome: Evidence from a host-free in vitro colonic model
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
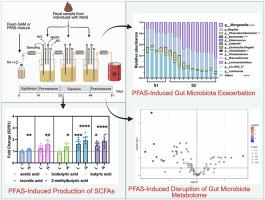
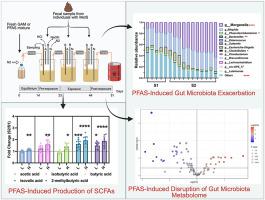
Metabolic syndrome (MetS) is a global health concern linked to metabolic abnormalities and increased risk of type 2 diabetes and cardiovascular disease. Recent studies suggest that exposure to per- and polyfluoroalkyl substances (PFAS) may contribute to MetS through alterations in gut microbiota and metabolism, but the underlying mechanisms remain unclear. This study aimed to investigate the effects of PFAS exposure on gut microbiota composition and metabolism in MetS using a three-stage, automated, computer-controlled in vitro Human Colonic Model (HCM) system. We introduced PFAS exposure to the gut microbiome in vitro at two concentrations (100 ng/mL and 1000 ng/mL) and analyzed microbial community structure using microbiome analysis, while changes in gut microbial metabolism were assessed through targeted and untargeted metabolomics. Our results showed that PFAS exposure significantly altered gut microbiota composition, with notable changes in key genera such as Morganella and Bilophila. Metabolomics analysis revealed an increase in short-chain fatty acid (SCFA) production at 1000 ng/mL of PFAS exposure, as well as significant alterations in other metabolites, including decreased acetophenone and taurocholic acid in both concentrations. These findings suggest that PFAS exposure may disrupt gut microbiota homeostasis and contribute to metabolic disturbances associated with MetS. This study highlights the need for further investigation into the mechanisms underlying PFAS-induced alterations in gut microbiota and their potential impact on human health.
全氟和多氟烷基物质(PFAS)在代谢综合征中破坏肠道微生物组组成和代谢:来自无宿主体外结肠模型的证据
代谢综合征(MetS)是一个全球性的健康问题,与代谢异常和2型糖尿病和心血管疾病风险增加有关。最近的研究表明,暴露于全氟烷基和多氟烷基物质(PFAS)可能通过改变肠道微生物群和代谢而导致MetS,但其潜在机制尚不清楚。本研究旨在研究PFAS暴露对MetS患者肠道微生物群组成和代谢的影响,采用三阶段、自动化、计算机控制的体外人类结肠模型(HCM)系统。我们在体外以两种浓度(100 ng/mL和1000 ng/mL)将PFAS暴露于肠道微生物组,并通过微生物组分析分析微生物群落结构,同时通过靶向和非靶向代谢组学评估肠道微生物代谢的变化。我们的研究结果表明,PFAS暴露显著改变了肠道微生物群的组成,其中关键属如摩根氏菌和Bilophila发生了显著变化。代谢组学分析显示,在1000 ng/mL的PFAS暴露下,短链脂肪酸(SCFA)的产生增加,其他代谢物也发生了显著变化,包括在两种浓度下苯乙酮和牛磺胆酸的减少。这些发现表明,PFAS暴露可能会破坏肠道微生物群的稳态,并导致与MetS相关的代谢紊乱。这项研究强调了进一步研究pfas诱导肠道微生物群改变的机制及其对人类健康的潜在影响的必要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


