Gram-scale synthesis of N-NiMo/MoO2 heterostructures to boost hydrogen evolution in low-alkalinity anion exchange membrane water electrolysis
IF 17.1
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Anion exchange membrane water electrolysis (AEMWE) presents a practical approach for green H2 production. However, the cathodic hydrogen evolution reaction (HER) in basic media is still sluggish as the lack of the cost-efficient catalysts for further industrialization. Here, the heterostructured catalysts combining nucleophilic and oxophilic components facilitate water dissociation and enhance HER and synthesized via a facile gel-route, featuring abundant interfaces between N-doped NiMo alloy and MoO2 particles. The heterostructured catalyst exhibits exceptional HER activity comparable to 60 %PtC catalysts in basic media (pH = 7–14), delivering high current densities of > 1 A cm−2 with long-term durability. AEMWE simulation demonstrates a low cell voltage of 1.87 V at 1 A cm−2, even with dilute 0.1 M KOH electrolyte. DFT calculations support the interfacial excitation that MoO2 clusters and N-doped NiMo alloy act synergistically to facilitate water dissociation and optimize *H binding, thereby accelerating hydrogen evolution kinetics. This work offers a practical approach for scalable fabrication of high performance, nonprecious-metal-based electrocatalysts for real application of AEMWE.
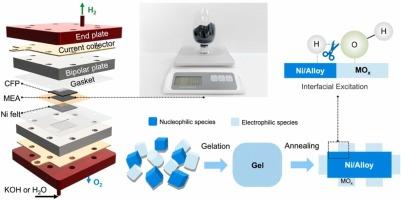
低碱度阴离子交换膜电解中N-NiMo/MoO2异质结构的克级合成促进析氢
阴离子交换膜电解(AEMWE)是一种实用的绿色制氢方法。然而,由于缺乏经济高效的催化剂,阴极析氢反应在基本介质中的发展仍然缓慢。本研究中,结合亲核和亲氧组分的异质结构催化剂促进了水的解离,提高了HER,并通过简单的凝胶途径合成,具有n掺杂NiMo合金与MoO2颗粒之间丰富的界面。异质结构催化剂在碱性介质(pH = 7~14)中表现出优异的HER活性,可与60%PtC催化剂相媲美,可提供>;1 A cm−2的高电流密度,并具有长期耐用性。AEMWE模拟表明,在1 a cm−2时,即使在0.1 M KOH溶液中,电池电压也低至1.87 V。DFT计算支持MoO2簇与n掺杂NiMo合金协同作用促进水解离和优化*H结合的界面激发,从而加速析氢动力学。这项工作为大规模制造高性能、非贵金属基电催化剂提供了一种实用的方法,可用于AEMWE的实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


