Sensory neuro-tumor crosstalk: Therapeutic opportunities and emerging frontiers in cancer neuroscience
IF 9.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Reviews on cancer
Pub Date : 2025-09-26
DOI:10.1016/j.bbcan.2025.189464
引用次数: 0
Abstract
Emerging evidence in cancer neuroscience highlights the crucial role of sensory nerves in tumor progression, an aspect of cancer pathobiology previously overlooked. Mechanistically, tumor-associated sensory neurons establish a self-reinforcing oncogenic loop via secreted neurotrophic factors (e.g., NGF/BDNF), which directly promote cancer cell growth, spread, and treatment resistance through Trk activation. Concurrently, tumors rewire their local environment through dysregulated expression of axon guidance molecules, facilitating invasive growth. Importantly, nociceptive signaling activated during perineural invasion not only mediates cancer-related pain but also shapes an immunosuppressive microenvironment through neuropeptide-mediated changes in immune cell function. Current therapeutic strategies targeting tumor-associated nerves focus on: (1) Pharmacological blockade of nerve-tumor communication using small-molecule inhibitors (e.g., Trk inhibitor larotrectinib); (2) Bioelectronic modulation of neural activity via modalities such as transcutaneous electrical nerve stimulation. Notably, preclinical models reveal enhanced efficacy when combining neural modulation with immune checkpoint inhibitors. Technological breakthroughs, including single-cell analysis for precise nerve targeting and advanced drug delivery systems, are improving therapeutic precision. Consequently, understanding the complex interactions between nerves and tumors requires integrated approaches combining cancer biology, neuroimmunology, and systems neuroscience. This conceptual shift not only reshapes our understanding of cancer pathophysiology but also opens new avenues for precision therapies aligned with modern oncology.
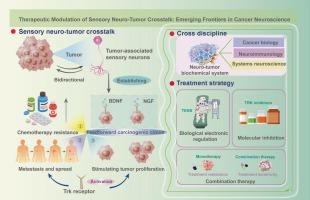
感觉神经肿瘤相声:癌症神经科学的治疗机会和新兴前沿。
癌症神经科学的新证据强调了感觉神经在肿瘤进展中的关键作用,这是以前被忽视的癌症病理生物学的一个方面。机制上,肿瘤相关感觉神经元通过分泌神经营养因子(如NGF/BDNF)建立自我强化的致癌环,通过Trk激活直接促进癌细胞生长、扩散和治疗抵抗。同时,肿瘤通过轴突引导分子的表达失调,重新连接其局部环境,促进侵袭性生长。重要的是,在神经周围侵袭过程中激活的伤害性信号不仅介导癌症相关疼痛,还通过神经肽介导的免疫细胞功能改变形成免疫抑制微环境。目前针对肿瘤相关神经的治疗策略主要集中在:(1)使用小分子抑制剂(如Trk抑制剂larorectinib)阻断神经-肿瘤通讯;(2)通过经皮神经电刺激等方式对神经活动进行生物电子调节。值得注意的是,临床前模型显示神经调节与免疫检查点抑制剂联合使用的疗效增强。技术突破,包括用于精确神经靶向的单细胞分析和先进的药物输送系统,正在提高治疗精度。因此,理解神经和肿瘤之间复杂的相互作用需要将癌症生物学、神经免疫学和系统神经科学结合起来。这种观念的转变不仅重塑了我们对癌症病理生理学的理解,而且为与现代肿瘤学相一致的精确治疗开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Reviews on cancer
医学-生化与分子生物学
CiteScore
17.20
自引率
0.00%
发文量
138
审稿时长
33 days
期刊介绍:
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer encompasses the entirety of cancer biology and biochemistry, emphasizing oncogenes and tumor suppressor genes, growth-related cell cycle control signaling, carcinogenesis mechanisms, cell transformation, immunologic control mechanisms, genetics of human (mammalian) cancer, control of cell proliferation, genetic and molecular control of organismic development, rational anti-tumor drug design. It publishes mini-reviews and full reviews.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


