Astragali radix - Curcumae rhizoma herb pair enhances Sorafenib's efficacy by inducing ferroptosis and activates Th1 cell immune response synergistically against hepatocellular carcinoma
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Sorafenib, as a first-line targeted drug for hepatocellular carcinoma (HCC), suffers from insufficient efficacy and dose-dependent toxic side effects, necessitating the development of combination therapy strategies. Clinical studies have shown that the Astragali radix - Curcumae rhizoma herb pair (ACHP) synergized with Sorafenib significantly enhanced the efficacy in advanced HCC patients, as well as improved immune function, but its synergistic mechanism remains unclear.
Purpose
This study aimed to reveal the mechanisms by which ACHP synergistically enhances Sorafenib's anti-HCC efficacy through a dual-mode of "ferroptosis -immunomodulation", and to provide a solid theoretical basis for its clinical application.
Methods
UPLC-Q-TOF-MS/MS was employed for the component characterization of ACHP extract. In vivo and in vitro HCC models were established to evaluate the efficacy and safety of ACHP synergized with Sorafenib against HCC. Transcriptome sequencing was utilized to screen potential molecular mechanisms, while molecular biology techniques, flow cytometry and inhibitors were applied to detect ferroptosis-related markers and Th1 cell-mediated immune response markers, aiming to reveal the underlying mechanisms.
Results
A total of 30 components were identified from ACHP extract. Pharmacodynamic evaluations showed that ACHP synergized with Sorafenib significantly suppressed tumor growth and cell proliferation of HCC, and exhibited favorable safety. Transcriptome sequencing suggested that the anti-HCC effect of ACHP synergized with Sorafenib might involve the induction of ferroptosis and modulation of Th1/Th2 cell differentiation. Further in vivo and in vitro experiments demonstrated that ACHP enhanced Sorafenib's efficacy by regulating the xCT/GPX4 and ACSL4/ALOX15 pathways to induce lipid peroxidation-related ferroptosis. Meanwhile, ACHP activated the IL-12/STAT4 signaling axis, promoted Th1 cell differentiation and up-regulated IFN-γ secretion, and further induced M1-type macrophages polarization and IL-12 secretion, thereby strengthening the IL-12-driven positive feedback immune loop.
Conclusion
ACHP enhances Sorafenib's efficacy by inducing lipid peroxidation-related ferroptosis and activates Th1-type anti-tumor immune responses, synergistically suppressing HCC. The study establishes a complete evidence chain of "component characterization-mechanism verification", providing novel therapeutic targets and strategic insights for the development of combination therapy for HCC.
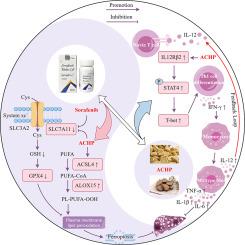
黄芪-姜黄对通过诱导铁凋亡和协同激活Th1细胞免疫应答增强索拉非尼抗肝癌的疗效。
背景:索拉非尼作为治疗肝细胞癌(HCC)的一线靶向药物,存在疗效不足和剂量依赖性毒副作用,需要发展联合治疗策略。临床研究表明,黄芪-姜黄对与索拉非尼协同作用可显著提高晚期HCC患者的疗效,并改善免疫功能,但其协同作用机制尚不清楚。目的:本研究旨在揭示ACHP通过“铁凋亡-免疫调节”双模式协同增强索拉非尼抗hcc疗效的机制,为其临床应用提供坚实的理论基础。方法:采用UPLC-Q-TOF-MS/MS法对石竹草提取物进行成分表征。建立体内和体外肝癌模型,评价acp与索拉非尼协同治疗肝癌的疗效和安全性。利用转录组测序技术筛选潜在的分子机制,利用分子生物学技术、流式细胞术和抑制剂技术检测凋亡相关标志物和Th1细胞介导的免疫应答标志物,旨在揭示其潜在的机制。结果:共鉴定出30种成分。药效学评价显示,与索拉非尼协同作用可显著抑制肝癌的肿瘤生长和细胞增殖,且具有良好的安全性。转录组测序提示,与索拉非尼协同的ACHP抗hcc作用可能与诱导铁凋亡和调节Th1/Th2细胞分化有关。进一步的体内和体外实验表明,ACHP通过调节xCT/GPX4和ACSL4/ALOX15通路诱导脂质过氧化相关的铁下垂,从而增强Sorafenib的疗效。同时,ACHP激活IL-12/STAT4信号轴,促进Th1细胞分化,上调IFN-γ分泌,进一步诱导m1型巨噬细胞极化和IL-12分泌,从而强化IL-12驱动的正反馈免疫回路。结论:ACHP通过诱导脂质过氧化相关铁下垂增强索拉非尼的疗效,激活th1型抗肿瘤免疫应答,协同抑制HCC。本研究建立了“组分表征-机制验证”的完整证据链,为HCC联合治疗的发展提供了新的治疗靶点和战略见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


