Suspensaditerpenes A–Q, anti-inflammatory labdane and nor-labdane diterpenoids from the seeds of Forsythia suspensa
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Phytochemical investigation of the seeds of Forsythia suspensa resulted in the isolation and characterization of 17 undescribed labdane and nor-labdane diterpenoids, named suspensaditerpenes A−Q (1−17), along with 13 known ones (18−30). Their structures were elucidated by a combination of NMR, HR-MS, and X-ray diffraction analyses. Structurally, 5, 10, and 13 are labdane diterpenoids featuring a lactone ring at the side chain, marking their first description in F. suspensa. Compounds 4, 14, 15, and 25 are 14,15-dinor-diterpenoids, among which 14 and 15 have a rare 6/6/5 ring skeleton. Furthermore, all known compounds were isolated from F. suspensa for the first time. The in vitro anti-inflammatory activities assay showed that 5, 8, 13, and 14 significantly inhibited the LPS-induced NO production at 20 μM in LPS-induced RAW264.7 cells, while the remaining 24 compounds demonstrated slight NO inhibitory activity. Further studies showed that 5 suppressed the LPS-induced expression of iNOS, p-p65, and NLRP3 proteins as well as NF-κB nuclear translocation in RAW264.7 cells.
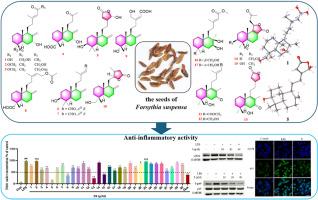
连翘种子中悬浮液萜类A-Q、抗炎液丹和非悬浮液丹二萜类。
对连翘(Forsythia suspensa)种子进行了植物化学研究,分离并鉴定了17种未描述的双萜和非双萜,命名为suspensaditerpenes A-Q(1-17),以及13种已知的双萜(18-30)。通过核磁共振、质谱和x射线衍射分析对其结构进行了鉴定。从结构上看,5、10和13是在侧链上有一个内酯环的唇丹二萜,这是它们在悬钩子中首次被描述。化合物4、14、15和25为14,15-二萜,其中14和15具有罕见的6/6/5环骨架。所有已知化合物均为首次从该菌中分离得到。体外抗炎活性实验结果显示,5、8、13和14明显抑制lps诱导的RAW264.7细胞20 μM的NO生成,其余24个化合物表现出轻微的NO抑制活性。进一步研究表明,5抑制lps诱导的RAW264.7细胞中iNOS、p-p65、NLRP3蛋白的表达以及NF-κB核易位。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


