A piRNA modulates the levels of 20-hydroxyecdysone in the ovary of the German cockroach
IF 2.3
2区 农林科学
Q1 ENTOMOLOGY
引用次数: 0
Abstract
PIWI-interacting RNAs (piRNAs) are small non-coding RNAs, typically 26 to 31 nucleotides long, originally known for silencing transposable elements (TEs), thus maintaining genomic stability. However, recent research has revealed additional regulatory roles. In this study, we investigate piRNA-305221, which is highly expressed in the ovaries of the German cockroach, Blattella germanica, to understand its involvement in oogenesis and reproduction. piRNA-305221 is found in germinal and somatic cells during the gonadotropic cycle, and is maternally provided to the egg. Its expression correlates with critical ovarian events, such as endoreplication and follicular cell differentiation, suggesting regulatory functions beyond TE silencing. Functional knockdown using antisense oligonucleotides (ASOs) resulted in delayed oviposition, malformed oothecae, and reduced offspring viability. Gene expression analysis revealed that the reduction of piRNA-305221 decreased shade (Cyp314a1) mRNA levels, impairing the conversion of ecdysone to its active form, 20-hydroxyecdysone, and a concomitant increase in expression of upstream steroidogenic genes (spook (Cyp307a1), phantom (Cyp306a1), disembodied (Cyp302a1)). These results indicate that piRNA-305221 may regulate steroidogenesis through direct or indirect control of mRNA targets. This study highlights the broader regulatory functions of piRNAs and demonstrates the utility of ASO-mediated knockdown in functional studies of non-coding RNAs.
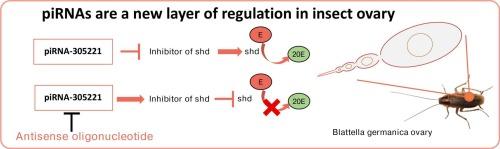
一种piRNA调节德国蟑螂卵巢中20-羟基蜕皮激素的水平。
piwi相互作用rna (pirna)是一种小的非编码rna,通常长26至31个核苷酸,最初被认为是沉默转座元件(te),从而维持基因组的稳定性。然而,最近的研究揭示了其他的调节作用。在这项研究中,我们研究了在德国小蠊卵巢中高表达的piRNA-305221,以了解其与卵发生和繁殖的关系。piRNA-305221存在于促性腺激素周期的生发细胞和体细胞中,并由母体提供给卵子。它的表达与关键的卵巢事件相关,如内膜复制和卵泡细胞分化,表明TE沉默之外的调节功能。使用反义寡核苷酸(ASOs)进行功能性敲除导致产卵延迟、卵囊畸形和后代生存能力降低。基因表达分析显示,piRNA-305221的减少降低了shade (Cyp314a1) mRNA水平,损害了蜕皮激素向其活性形式20-羟基蜕皮激素的转化,并同时增加了上游类固醇基因(spook (Cyp307a1), phantom (Cyp306a1), disembodied (Cyp302a1))的表达。这些结果表明piRNA-305221可能通过直接或间接控制mRNA靶点来调节甾体生成。这项研究强调了pirna更广泛的调控功能,并证明了aso介导的敲低在非编码rna的功能研究中的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of insect physiology
生物-昆虫学
CiteScore
4.50
自引率
4.50%
发文量
77
审稿时长
57 days
期刊介绍:
All aspects of insect physiology are published in this journal which will also accept papers on the physiology of other arthropods, if the referees consider the work to be of general interest. The coverage includes endocrinology (in relation to moulting, reproduction and metabolism), pheromones, neurobiology (cellular, integrative and developmental), physiological pharmacology, nutrition (food selection, digestion and absorption), homeostasis, excretion, reproduction and behaviour. Papers covering functional genomics and molecular approaches to physiological problems will also be included. Communications on structure and applied entomology can be published if the subject matter has an explicit bearing on the physiology of arthropods. Review articles and novel method papers are also welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


