Dihydroartemisinin inhibits histone lactylation through YAP1 to act as a ‘hot’ switch for ‘cold’ tumor in hepatocellular carcinoma
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Hepatocellular carcinoma (HCC) is characterized by a ‘cold’ tumor microenvironment (TME), which limits the efficacy of immune checkpoint inhibitors (ICIs). In a previous study, we demonstrated that dihydroartemisinin (DHA) could disrupt the immunosuppressive TME and delay HCC progression.
Purpose
This study aimed to explore the precise mechanisms underlying the shift of the TME from an immunosuppressive to an immunostimulatory state.
Methods
We constructed HCC subcutaneous allograft mice model to investigate the in vivo antitumor effects of DHA. We further performed single-cell RNA sequencing (scRNA-seq) and multiplex immunohistochemistry (mIHC) to analyze the immune dynamics during DHA-induced TME transition in HCC. Using NCG mice (lacking T, B, and NK cells), BALB/c nude mice (lacking T cells), and CD8+ T cell-depleted mice, we assessed whether the antitumor effect of DHA in HCC depends on its immune functions and determined the key role of T cells in antitumor immunity. Additional downstream mechanisms were explored using YAP1 knockdown HCC cells and liver-specific Yap1 knockout mice through techniques such as Western blot, immunofluorescence (IF), metabolic flux analysis, co-immunoprecipitation (Co-IP), chemokine chip, and flow cytometry (FCM).
Results
We observed that DHA transforms the TME of HCC from an immune-cold to an immune-hot state by increasing the abundance and proportion of T cells, NK cells, M1-like TAMs, and DCs. Notably, DHA was found to enhance the proportion of IFN-γ+ CD8+ T cells within the TME of HCC-bearing mice, and its therapeutic effects were dependent on CD8+ T cells. Our results suggest that DHA could suppress histone lactylation (Kla) and acetylation (Kac) in HCC cells by inhibiting YAP1. Mechanistically, DHA reduces lactate transport, thereby decreasing lactate accumulation within the TME, lowering P300/CBP catalytic activity, and enhancing the deacetylation effect of HDAC1-3. This finding reveals a new mechanism by which DHA, through metabolic changes and epigenetic regulation, remodels the TME of HCC. Additionally, a combination strategy involving DHA and anti-PD-1 therapy demonstrated the potential to reshape the cold HCC TME via inhibition of the YAP1-lactylation positive feedback loop.
Conclusion
Our study confirms that DHA inhibits histone Kla in HCC cells through YAP1 inhibition, which shifts the TME from an immune-cold to immune-hot state. Moreover, DHA enhance the anti-HCC immune response dependent on CD8+ T cells, thereby sensitizing anti-PD-1 therapy. This novel finding positions DHA as a promising strategy for optimizing anti-PD-1 therapy in HCC, warranting further clinical application studies.
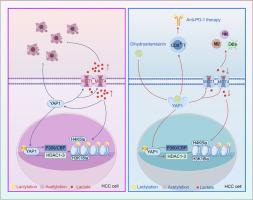
双氢青蒿素通过YAP1抑制组蛋白乳酸化,在肝细胞癌中充当“冷”肿瘤的“热”开关。
背景:肝细胞癌(HCC)以“冷”肿瘤微环境(TME)为特征,这限制了免疫检查点抑制剂(ICIs)的疗效。在之前的一项研究中,我们证明了双氢青蒿素(DHA)可以破坏免疫抑制的TME并延缓HCC的进展。目的:本研究旨在探讨TME从免疫抑制状态向免疫刺激状态转变的确切机制。方法:建立肝癌皮下移植小鼠模型,观察DHA的体内抗肿瘤作用。我们进一步进行了单细胞RNA测序(scRNA-seq)和多重免疫组织化学(mIHC)来分析dha诱导的HCC中TME转移的免疫动力学。利用NCG小鼠(缺乏T、B和NK细胞)、BALB/c裸鼠(缺乏T细胞)和CD8+ T细胞缺失小鼠,我们评估了DHA在HCC中的抗肿瘤作用是否取决于其免疫功能,并确定了T细胞在抗肿瘤免疫中的关键作用。通过Western blot、免疫荧光(IF)、代谢通量分析、共免疫沉淀(Co-IP)、趋化因子芯片和流式细胞术(FCM)等技术,研究了YAP1敲除HCC细胞和肝脏特异性YAP1敲除小鼠的其他下游机制。结果:我们观察到DHA通过增加T细胞、NK细胞、m1样tam和dc的丰度和比例,将HCC的TME从免疫冷状态转变为免疫热状态。值得注意的是,DHA可提高hcc小鼠TME中IFN-γ+ CD8+ T细胞的比例,其治疗作用依赖于CD8+ T细胞。我们的研究结果表明DHA可以通过抑制YAP1抑制HCC细胞的组蛋白乳酸化(Kla)和乙酰化(Kac)。在机制上,DHA减少乳酸转运,从而减少乳酸在TME内的积累,降低P300/CBP的催化活性,增强HDAC1-3的去乙酰化作用。这一发现揭示了DHA通过代谢变化和表观遗传调控重塑HCC TME的新机制。此外,DHA和抗pd -1治疗的联合策略表明,通过抑制yap1 -乳酸化正反馈回路,有可能重塑冷HCC TME。结论:我们的研究证实,DHA通过抑制YAP1抑制HCC细胞中的组蛋白Kla,使TME从免疫冷状态转变为免疫热状态。此外,DHA增强了依赖于CD8+ T细胞的抗hcc免疫应答,从而使抗pd -1治疗变得敏感。这一新发现表明DHA是优化HCC抗pd -1治疗的一种有前景的策略,值得进一步的临床应用研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


