Construction of a modified Arrhenius equation for predicting drug nitrosation in solid dosage form
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
To prevent contamination by nitrosamine drug substance-related impurities (NDSRIs) in solid dosage forms, the modeling of drug nitrosation reactions was investigated. The nitrosation reaction rate of desloratadine (DES) in the physical mixtures (PMs) was measured under various temperature, relative humidity, and alkaline excipient (AE) content conditions. The nitrosation reaction rate increased with increasing relative humidity and temperature. In contrast, an increase in AE content resulted in a decreased nitrosation reaction rate. The relationship between these three conditions and reaction rate was analyzed by multiple regression analysis using the least-squares method. The modified Arrhenius equation was expressed as ln k = 41.38 −13026 × (1/T) + 0.038 × (%RH) − 0.44 × (% w/w(AE)). The values predicted by the modified Arrhenius equation were in good agreement with the experimental values, indicating that the model equation was valid. The prediction accuracy of the modified Arrhenius equation was higher than that of the original Arrhenius equation. The modified Arrhenius equation can be applied to the nitrosation reactions of various drugs and is expected to improve the quality, safety, and efficiency of drug research, development, and manufacturing processes.
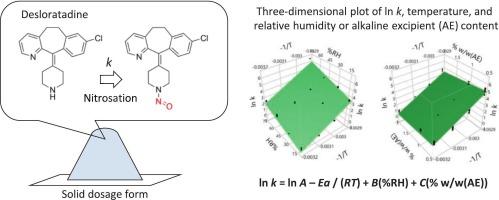
预测固体剂型药物亚硝化的修正Arrhenius方程的建立。
为了防止固体剂型中亚硝胺类药物相关杂质(NDSRIs)的污染,研究了药物亚硝化反应的模型。测定了不同温度、相对湿度和碱性辅料含量条件下地氯雷他定(DES)在物理混合物(PMs)中的亚硝化反应速率。随着相对湿度和温度的升高,亚硝化反应速率增大。相反,AE含量的增加导致亚硝化反应速率降低。采用最小二乘法对这三种条件与反应速率的关系进行多元回归分析。修正后的Arrhenius方程表示为ln k = 41.38 -13026 × (1/T) + 0.038 × (%RH) - 0.44 × (% w/w(AE))。修正Arrhenius方程的预测值与实验值吻合较好,表明模型方程是有效的。修正后的Arrhenius方程预测精度高于原Arrhenius方程。修正后的Arrhenius方程可应用于多种药物的亚硝化反应,有望提高药物研发和生产过程的质量、安全性和效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


