Emodin facilitates the transformation of A1/A2 reactive astrocytes through the 11β-HSD1/AKT signaling pathway in the context of cerebral ischemia
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Emodin is the main active ingredient of Rhei Radix et Rhizoma, a herb widely used for ischemic stroke treatment. This study demonstrates for the first time that emodin exerts neuroprotective effects against ischemic stroke by regulating astrocyte phenotypic transformation via the 11β-HSD1/AKT signaling pathway. In rat MCAO and astrocyte/neuron OGD/R models, emodin improved neurological function, reduced infarction volume, and shifted astrocytes from detrimental A1 to beneficial A2 phenotypes. Mechanistically, emodin activated AKT phosphorylation while suppressing the NF-κB pathway (reducing p-IκBα/IκBα and p-p65/p65 ratios). These effects—along with functional improvements—were reversed by AKT inhibitor LY294002. Furthermore, 11β-HSD1 siRNA mimicked emodin's actions in vitro, and supernatants from both emodin- and siRNA-treated astrocytes enhanced neuronal MAP2 expression. These findings identify astrocyte phenotypic modulation via 11β-HSD1/AKT as a key therapeutic mechanism of emodin.
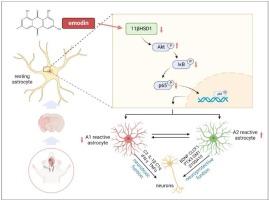
在脑缺血情况下,大黄素通过11β-HSD1/AKT信号通路促进A1/A2反应性星形胶质细胞的转化。
大黄素是大黄的主要活性成分,是一种广泛用于缺血性中风治疗的草药。本研究首次证实大黄素通过11β-HSD1/AKT信号通路调控星形细胞表型转化,对缺血性脑卒中具有神经保护作用。在大鼠MCAO和星形细胞/神经元OGD/R模型中,大黄素改善了神经功能,减少了梗死体积,并将星形细胞从有害的A1表型转变为有益的A2表型。机制上,大黄素激活AKT磷酸化,同时抑制NF-κB通路(降低p -κB α/ i -κB α和p-p65/p65比率)。AKT抑制剂LY294002可以逆转这些影响以及功能改善。此外,11β-HSD1 siRNA在体外模拟了大黄素的作用,大黄素和siRNA处理的星形胶质细胞的上清液增强了神经元MAP2的表达。这些发现表明,通过11β-HSD1/AKT调节星形胶质细胞表型是大黄素的关键治疗机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


