Real-time monitoring and characterization of thin film drug delivery systems using optical coherence tomography
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The development of drug delivery systems demands close attention to product quality and consistency of quality attributes to guarantee reproducibility in fabrication, performance and therapeutic effectiveness. While some methods exist for monitoring in-line manufacturing, these methods are limited in terms of spatial resolution or depth penetration, which can lead to inconsistent characterization. Furthermore, conventional static characterization methods often lack the ability to monitor time-dependent changes within drug delivery systems. This limits their effectiveness in capturing dynamic processes such as swelling, disintegration, and drug release, underscoring the need for advanced techniques that offer reliable, high-resolution, and depth-specific insights into these mechanisms over time. Optical coherence tomography (OCT) is a non-invasive, high-resolution imaging technique which shows promise for comprehensive characterization of structural and dynamic characterization of drug delivery systems. In this study, we explore applications of OCT to structural and dynamic characterization of thin film drug delivery systems. We demonstrate that OCT can be used to non-destructively monitor the spatial variation of the refractive index and thickness of thin films, providing crucial feedback for quality control and ensuring standardized doses in individual film units. Further, we demonstrate that OCT can be used to monitor the dissolution dynamics, enabling characterization of time-controlled release mechanisms. These results suggest that OCT has the potential to enhance thin-film characterization by enabling more precise monitoring of critical quality attributes, which can contribute to improved manufacturing consistency and better control over the uniformity of the dose in each film unit.
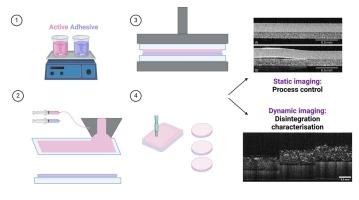
利用光学相干断层成像技术对薄膜药物输送系统进行实时监测和表征。
药物输送系统的开发需要密切关注产品质量和质量属性的一致性,以保证制造、性能和治疗效果的可重复性。虽然存在一些监测在线制造的方法,但这些方法在空间分辨率或深度穿透方面受到限制,这可能导致表征不一致。此外,传统的静态表征方法往往缺乏监测药物输送系统内随时间变化的能力。这限制了它们捕捉诸如肿胀、崩解和药物释放等动态过程的有效性,强调了对先进技术的需求,这些技术可以随着时间的推移提供可靠、高分辨率和深度特定的见解。光学相干断层扫描(OCT)是一种非侵入性、高分辨率的成像技术,有望全面表征药物输送系统的结构和动态特征。在这项研究中,我们探索了OCT在薄膜给药系统结构和动力学表征中的应用。我们证明OCT可以用于非破坏性地监测薄膜的折射率和厚度的空间变化,为质量控制和确保单个薄膜单元的标准化剂量提供关键反馈。此外,我们证明OCT可以用于监测溶解动力学,从而表征时间控制的释放机制。这些结果表明,OCT具有通过更精确地监测关键质量属性来增强薄膜表征的潜力,这有助于提高制造一致性和更好地控制每个膜单元中剂量的均匀性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


