“Single molecule tracking unveils distinct FcγRIIB and FcRn plasma membrane dynamics”
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
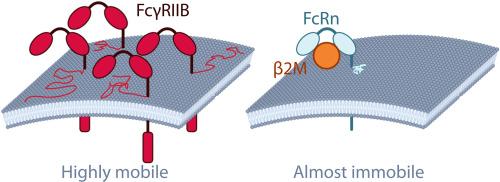
Fc receptors (FcRs) constitute a heterogeneous family of membrane-bound proteins that are integral to the immune system function, primarily through their ability to bind the constant domain (Fc) of antibodies. These receptors mediate a variety of immunological processes and maintain immunological homeostasis. FcRs exhibit a broad distribution throughout the body, being expressed not only on the surface of various immune cells but also in non-immune cells and tissues. Over the past decade, specific FcRs -most notably the neonatal Fc receptor (FcRn) and Fc gamma receptor IIb (FcγRIIB)- have emerged as putative mediators in processes such as antibody-mediated uptake. However, the literature presents conflicting evidence regarding their precise roles and mechanisms in antibody internalization. In this study, we employed single molecule tracking in living epithelial-like cells to investigate the cellular distribution and dynamics of FcγRIIB and FcRn, basic features that are still largely uncharacterized. Our findings revealed that FcγRIIB, mostly present on the cell membrane, is highly mobile and can be actively internalized. Conversely, FcRn was primarily localized intracellularly, with only a minor fraction capable of reaching the cell surface, where it exhibited minimal mobility. These results show that FcγRIIB, but not FcRn, displays the basic expected requisites that would be necessary for surface antibody binding and uptake.
“单分子追踪揭示了不同的FcγRIIB和FcRn质膜动力学”。
Fc受体(FcRs)构成了一个异质的膜结合蛋白家族,主要通过其结合抗体恒定结构域(Fc)的能力,对免疫系统功能至关重要。这些受体介导多种免疫过程并维持免疫稳态。FcRs在全身分布广泛,不仅在各种免疫细胞表面表达,也在非免疫细胞和组织中表达。在过去的十年中,特异性fcr -最显著的是新生儿Fc受体(FcRn)和Fcγ受体IIb (Fcγ riib)-已被认为是抗体介导摄取等过程中的介质。然而,关于它们在抗体内化中的确切作用和机制,文献提出了相互矛盾的证据。在本研究中,我们在活的上皮样细胞中采用单分子跟踪来研究FcγRIIB和FcRn的细胞分布和动力学,这些基本特征在很大程度上仍未被表征。我们的研究结果表明,fc - γ riib主要存在于细胞膜上,具有高度的流动性,可以主动内化。相反,FcRn主要定位于细胞内,只有一小部分能够到达细胞表面,在那里它表现出最小的流动性。这些结果表明,FcγRIIB,而不是FcRn,显示了表面抗体结合和摄取所必需的基本条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


