The immunologic and stromal functions of nitric oxide in fibrotic tumor microenvironments: A mini-review
IF 2.7
Advances in redox research : an official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe
Pub Date : 2025-09-26
DOI:10.1016/j.arres.2025.100139
引用次数: 0
Abstract
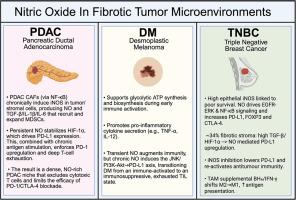
Tumors characterized by a prominent desmoplastic stroma – including pancreatic ductal adenocarcinoma, the desmoplastic melanoma subtype, and a subset of triple-negative breast cancer feature a dense, collagen-rich stroma that impairs drug penetration, skews myeloid cellular function, and either excludes, permits, or exhausts effective lymphocyte function. Nitric oxide sits at the center of this microenvironmental interchange. To delineate nitric oxide’s dual functions, this study surveyed mechanistic and translational studies on NO signaling in fibrotic tumor microenvironments indexed in PubMed and Web of Science through 2025. In pancreatic ductal adenocarcinoma, chronic inducible nitric oxide synthase activity within cancer-associated fibroblasts, tumor cells, and myeloid-derived suppressor cells stabilizes HIF-1α, drives PD-L1 expression, and reinforces a self-perpetuating loop of T-cell dysfunction. In desmoplastic melanoma, sustained nitric oxide flux may converge on JNK and PI3K/Akt dependent PD-L1 upregulation, fostering adaptive resistance. In triple negative breast cancer, Roughly 34 % develop a fibrotic stroma where inducible nitric oxide synthase overexpression predicts poor survival. This poor survival is reflected in the highly fibrotic, immune-excluded milieu of TNBC with high TGF-β and HIF-1α activity. Like PDAC, sustained nitric oxide flux further stabilizes HIF-1α, amplifying hypoxia responsive gene programs and reinforcing stromal fibrosis. Collectively, these findings reveal a concentration, isoform, and context-specific spectrum of nitric oxide activity: pathologic high output inducible nitric oxide synthase-derived flux promotes immunosuppression and metastasis, whereas basal or controlled nitric oxide levels supports vascular integrity and, in some contexts, antitumor immunity. Therapeutically, a multifaceted approach combining inducible nitric oxide inhibition, calibrated nitric oxide donors, myeloid-derived suppressor cell inhibition, and tumor associated macrophage repolarization with immune checkpoint inhibitors offers a precision framework to dismantle fibrotic stromal barriers and convert immune-cold desmoplastic cancers into responsive disease.
一氧化氮在纤维化肿瘤微环境中的免疫和间质功能:综述
以显著的间质增生为特征的肿瘤,包括胰腺导管腺癌、间质增生黑色素瘤亚型和三阴性乳腺癌的一个亚群,其特征是致密、富含胶原的间质损害药物渗透,扭曲髓细胞功能,排除、允许或耗尽有效淋巴细胞功能。一氧化氮位于这个微环境交换的中心。为了描述一氧化氮的双重功能,本研究调查了截至2025年PubMed和Web of Science收录的纤维化肿瘤微环境中NO信号传导的机制和转化研究。在胰腺导管腺癌中,癌症相关成纤维细胞、肿瘤细胞和髓源性抑制细胞中的慢性诱导型一氧化氮合酶活性稳定HIF-1α,驱动PD-L1表达,并加强t细胞功能障碍的自我延续循环。在粘连性黑色素瘤中,持续的一氧化氮通量可能会聚在JNK和PI3K/Akt依赖的PD-L1上调上,从而促进适应性抵抗。在三阴性乳腺癌中,大约34%的患者发展为纤维化基质,诱导型一氧化氮合酶过表达预示着较差的生存率。这种低生存率反映在TNBC高度纤维化,免疫排斥的环境中,具有高TGF-β和HIF-1α活性。与PDAC一样,持续的一氧化氮通量进一步稳定了HIF-1α,放大了缺氧反应基因程序并加强了间质纤维化。总的来说,这些发现揭示了一氧化氮活性的浓度、异构体和环境特异性谱:病理性高输出诱导型一氧化氮合酶衍生的通量促进免疫抑制和转移,而基础或控制的一氧化氮水平支持血管完整性,并在某些情况下支持抗肿瘤免疫。在治疗上,结合诱导型一氧化氮抑制、校准型一氧化氮供体、髓源性抑制细胞抑制和肿瘤相关巨噬细胞复极化与免疫检查点抑制剂的多方面方法,提供了一个精确的框架来拆除纤维化间质屏障,并将免疫冷性结缔组织增生癌转化为反应性疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advances in redox research : an official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe
Biochemistry, Genetics and Molecular Biology (General)
CiteScore
2.60
自引率
0.00%
发文量
0
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


