Copper-Catalyzed Diastereo- and Enantioselective 1,3-Dipolar Cycloaddition of Imino Lactones with Ylidene-Pyrazolones
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A Cu(MeCN)4BF4/Fesulphos complex successfully catalyzes the asymmetric 1,3-dipolar cycloaddition (1,3-DC) of imino lactones with 4-ylidene-pyrazol-5-ones to afford bis-spirocyclic pyrrolidines in good yields with high diastereo- and enantioselectivities (up to >20:1 dr, 99% ee). In addition, exo'-(2,5-trans-4,5-trans)-pyrrolidine scaffolds are exclusively produced as single diastereomers. A wide variety of imino lactones bearing electron-donating and electron-withdrawing groups on the phenyl groups and heteroaryl substrates is employed in this reaction. The substrate scope of ylidene-pyrazol-5-ones is investigated, and the substituents on the phenyl ring have limited effect on the product yields and stereoselectivity of the reaction.
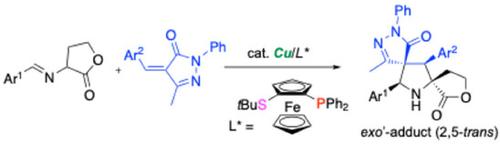
铜催化酰基吡唑酮与亚胺内酯的非映对和对映选择性1,3-偶极环加成反应
Cu(MeCN)4BF4/Fesulphos配合物成功催化亚胺内酯与4-酰基-吡唑-5-酮的不对称1,3-偶极环加成反应(1,3- dc)得到双螺环吡咯烷,产率高,非映对和对映选择性高(高达20:1 dr, 99% ee)。此外,外展'-(2,5-反式-4,5-反式)-吡咯烷支架仅以单一非对映体的形式生产。各种各样的亚胺内酯在苯基和杂芳基底物上带有供电子和吸电子基团,用于该反应。研究了吡啶-吡唑-5-酮反应的底物范围,发现苯基环上的取代基对反应的产率和立体选择性影响有限。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


