Highly potent and selective degrader targeting ERα to improve the treatment outcomes of breast cancer
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Estrogen receptor alpha (ERα) is overexpressed in approximately 70 % of breast cancer cases; therefore, it is considered a primary therapeutic target for breast cancer. Several therapeutic agents, including selective estrogen receptor modulators, aromatase inhibitors, selective estrogen receptor degraders, and proteolysis-targeting chimeras (PROTACs), have been developed to antagonize and degrade ERα. The representative ERα-targeting PROTAC (ERα-PROTAC) agent ARV-471 has been used to treat locally advanced or metastatic breast cancer in clinical trials. Herein, we designed, synthesized, and evaluated several novel ERα-PROTAC agents. After systematic structural optimization, compound A16 was found to have excellent antiproliferative and ERα-inhibitory activities in the breast cancer cell line MCF-7. A16 selectively degraded ERα (DC50 = 3.78 nM) through the ubiquitin–proteasome pathway in a time- and concentration-dependent manner. It effectively attenuated drug resistance (MCF-7 Y537S cells; IC50 = 1.3 nM), inhibited proliferation, and induced apoptosis in MCF-7 cells. In addition, it exhibited excellent antitumor effects (10 mg/kg/d intraperitoneal injection; total growth inhibition = 80.11 %) and a good safety profile in an MCF-7 xenograft model, highlighting its potential as a novel drug candidate for breast cancer.
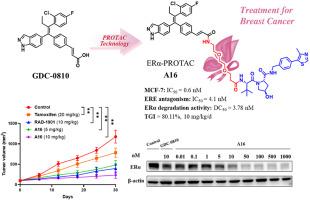
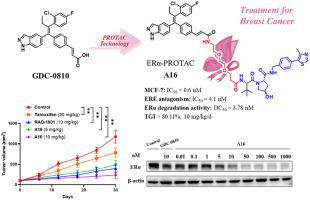
靶向ERα的高效选择性降解剂改善乳腺癌治疗效果
雌激素受体α (ERα)在大约70%的乳腺癌病例中过度表达;因此,它被认为是乳腺癌的主要治疗靶点。一些治疗药物,包括选择性雌激素受体调节剂、芳香酶抑制剂、选择性雌激素受体降解剂和靶向蛋白水解嵌合体(PROTACs),已经被开发出来对抗和降解ERα。具有代表性的er α靶向PROTAC (ERα-PROTAC)药物ARV-471已在临床试验中用于治疗局部晚期或转移性乳腺癌。在此,我们设计、合成并评价了几种新的ERα-PROTAC药物。经过系统的结构优化,发现化合物A16对乳腺癌细胞系MCF-7具有良好的抗增殖和er α-抑制活性。A16通过泛素-蛋白酶体途径选择性降解ERα (DC50 = 3.78 nM),且具有时间和浓度依赖性。有效降低MCF-7 Y537S细胞耐药,IC50 = 1.3 nM,抑制MCF-7细胞增殖,诱导细胞凋亡。此外,它还具有出色的抗肿瘤作用(10 mg/kg/d腹腔注射,总生长抑制率为80.11%),并且在MCF-7异种移植模型中具有良好的安全性,突出了其作为乳腺癌新型候选药物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


