Deep Learning-Driven Co-Assembly of Naturally Sourced Compound Nanoparticles for Potentiated Cancer Immunotherapy
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Co-assembly of excipient-free nanoparticles has emerged as a promising drug delivery platform due to their high drug-loading capacity, ease of preparation, and ability to achieve combination therapeutic effects. However, the absence of systematic design strategies has hindered their broader application. In this study, a deep learning platform, Gramord, is developed to rationally design the excipient-free anti-tumor nanoparticles of nature-sourced compounds. A comprehensive database of excipient-free nanoparticles is first built and used to train Gramord for predicting self-assembly compatibility. By screening 1800 naturally-derived small molecules and their derivatives, the compound pairs capable of forming excipient-free nanoparticles are identified. Leveraging the advantage of oridonin (Ori) for inducing apoptosis of tumor cells and cepharanthine (Cep) for eliciting immunogenic cell death of tumor cells, the Ori-Cep pair for preparing the self-assemble nanoparticles (namely OCN) is subsequently selected. Using a mouse model of CT26 colorectal tumor, it is demonstrated that the systemically administrated OCN specifically accumulate at the tumor sites, and regress tumor growth by inducing anti-tumor immunogenicity and recruiting tumor-infiltrating cytotoxic T lymphocytes. This study highlights the application of artificial intelligence in designing excipient-free nanomedicine, offering a scalable and cost-effective approach to expanded therapeutic options.
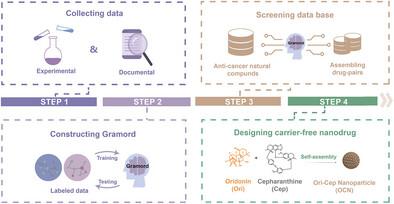
深度学习驱动的天然复合纳米颗粒协同组装用于增强癌症免疫治疗
无赋形剂纳米颗粒的共组装由于其高载药能力、易于制备和能够实现联合治疗效果而成为一种有前途的药物递送平台。然而,缺乏系统的设计策略阻碍了它们的广泛应用。在本研究中,我们开发了一个深度学习平台,Gramord,来合理设计无赋形剂的天然化合物抗肿瘤纳米颗粒。首先建立了一个无赋形剂纳米粒子的综合数据库,并用于训练Gramord预测自组装兼容性。通过筛选1800个天然衍生的小分子及其衍生物,鉴定了能够形成无赋形剂纳米颗粒的化合物对。利用oridonin (Ori)诱导肿瘤细胞凋亡和cepharanthine (Cep)诱导肿瘤细胞免疫原性死亡的优势,随后选择Ori-Cep对制备自组装纳米颗粒(即OCN)。通过小鼠CT26结直肠肿瘤模型,研究表明,全身给药OCN特异性地积聚在肿瘤部位,并通过诱导抗肿瘤免疫原性和招募肿瘤浸润的细胞毒性T淋巴细胞来抑制肿瘤生长。本研究强调了人工智能在设计无赋形剂纳米药物中的应用,为扩大治疗选择提供了一种可扩展且具有成本效益的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


