Dual Metabolic Rewiring Amplifies Cellular Oxidation and Reverses Immunosuppression for Combating Orthotopic Triple-Negative Breast Cancer
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
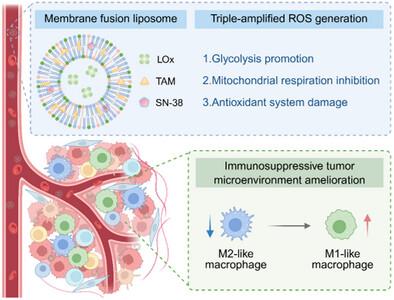
Abnormal glucose/lactate metabolism is one of the key hallmarks of a tumor. Leveraging the excessive metabolite lactate to generate reactive oxygen species (ROS) by lactate oxidase (LOx) has become an efficient method for cancer therapy. However, the hypoxic tumor microenvironment (TME) and ROS-scavenging enzymes largely restrict the catalytic activity of LOx. Herein, we design an ROS generator composed of LOx-encapsulated and 7-ethyl-10-hydroxycamptothecin (SN-38)- and tamoxifen (TAM)-coloaded membrane fusion liposomes, which can directly release cargoes via fusion with cancer cell membranes. Through the glycolysis promotion and mitochondrial respiration inhibition by TAM, and the antioxidant enzyme downregulation by SN-38, the generator not only spares oxygen and increases intracellular lactate for LOx catalysis to yield cytotoxic H2O2, but also avoids ROS removal, realizing triple-amplified ROS production and effective cancer cell killing. In the TME of an orthotopic breast cancer model, the generator consumes lactate and produces immunostimulatory H2O2, thereby promoting the M1-like polarization of tumor-associated macrophages and suppressing regulatory T cells to remodel the immunosuppressive TME. Additionally, the generator inhibits angiogenesis and further cancer metastasis via hypoxia inducible factor-1α (HIF-1α) signaling blockage. Collectively, this work provides an inspirational example of using a glucose/lactate metabolism interference strategy to fight against orthotopic triple-negative breast cancer.
双代谢重布线放大细胞氧化和逆转免疫抑制对抗原位三阴性乳腺癌
葡萄糖/乳酸代谢异常是肿瘤的关键标志之一。利用过量代谢产物乳酸通过乳酸氧化酶(LOx)产生活性氧(ROS)已成为癌症治疗的有效方法。然而,低氧肿瘤微环境(TME)和ros清除酶在很大程度上限制了LOx的催化活性。本研究设计了一种由lox包封的7-乙基-10-羟基喜树碱(SN-38)和他莫昔芬(TAM)包封的膜融合脂质体组成的ROS产生器,该脂质体可以通过与癌细胞膜融合直接释放产物。通过TAM促进糖酵解和抑制线粒体呼吸,通过SN-38下调抗氧化酶,不仅可以储备氧气,增加细胞内乳酸用于LOx催化产生细胞毒性H2O2,还可以避免去除ROS,实现三倍扩增ROS的产生,有效杀伤癌细胞。在原位乳腺癌模型TME中,该发生器消耗乳酸,产生免疫刺激性H2O2,从而促进肿瘤相关巨噬细胞m1样极化,抑制调节性T细胞,重塑免疫抑制性TME。此外,该发生器通过缺氧诱导因子-1α (HIF-1α)信号通路阻断抑制血管生成和进一步的癌症转移。总的来说,这项工作为使用葡萄糖/乳酸代谢干扰策略对抗原位三阴性乳腺癌提供了一个鼓舞人心的例子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


