Surface hydroxyl-driven =FeOFe(II)+ and =FeOH2+ generation on polyphenol-modified ZVI: Mechanistic insights into Cr(VI) and surface passivation layer removal
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
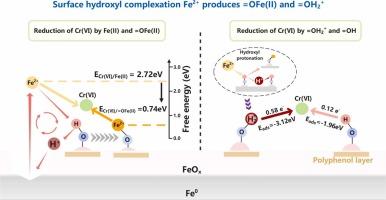
Micron zero-valent iron (mZVI) has been widely employed for heavy metal remediation. However, its surface oxide layer severely restricts interfacial electron transfer, resulting in low decontamination efficiency. Herein, a tannic acid-modified mZVI (TA-ZVI) was developed using ball milling. The modified material exhibited excellent Cr(VI) removal efficiency (98.2% in 2 min) due to the abundant presence of surface hydroxyl groups. The results of electrochemical analysis demonstrated that the hydroxyl groups increased the electron density and conductivity of the material surface, accelerated the electron transfer, and led to the formation of low and uniform surface potential on the material surface. The hydroxylated interface facilitated in situ Fe(II) generation and release to the solution. The hydroxyl groups then complexed with Fe(II) in the solution to form =FeOFe(II)+, which facilitated protonic reactions and led to the formation of =FeOH2+. Density functional theory (DFT) calculation proved that the reaction energy barrier of =FeOFe(II)+ for Cr(VI) was much smaller than that of Fe(II) in the solution. Moreover, the surface hydroxyl species (=FeOH and =FeOH2+) directly participated in Cr(VI) removal through synergistic adsorption-reduction pathways, thereby contributing significantly to the overall removal. This work reveals the removal mechanism of Cr(VI) by surface-hydroxylated ZVI, which is of great significance for the treatment of heavy metal ion pollution.
多酚修饰ZVI表面羟基驱动=FeOFe(II)+和=FeOH2+生成:Cr(VI)和表面钝化层去除的机理
微米零价铁(mZVI)在重金属修复中得到了广泛应用。但其表面氧化层严重限制了界面电子传递,导致去污效率低。采用球磨法制备了单宁酸改性mZVI (TA-ZVI)。改性后的材料由于表面羟基的大量存在,表现出优异的Cr(VI)去除率(2 min内98.2%)。电化学分析结果表明,羟基增加了材料表面的电子密度和电导率,加速了电子传递,导致材料表面形成低而均匀的表面电位。羟基化界面有利于原位Fe(II)生成和释放到溶液中。然后羟基与溶液中的Fe(II)络合形成=FeOFe(II)+,促进质子反应,形成=FeOH2+。密度泛函理论(DFT)计算证明,溶液中Cr(VI)的反应能垒=FeOFe(II)+远小于Fe(II)的反应能垒。此外,表面羟基(=FeOH和=FeOH2+)通过协同吸附-还原途径直接参与Cr(VI)的去除,从而对整体去除有显著贡献。本工作揭示了表面羟基化ZVI对Cr(VI)的去除机理,对重金属离子污染的治理具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


