Sequential separation and recovery of Li, Mn, Co, and Ni from spent lithium-ion batteries using integrated solvent extraction and precipitation
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The growing demand for lithium and critical elements in lithium-ion battery (LIB) production highlights the urgent need for recycling to support low-carbon transport and energy storage. Here, we present an integrated hydrometallurgical process combining optimized acid leaching with a multi-stage separation cascade, achieving > 98 % recovery of Li, Co, Mn, and Ni from spent LIB cathodes. Almost quantitative leaching of Li, Co, Mn and Ni from LIB powder was achieved utilizing 3.0 M H2SO4 with 10 vol% H2O2 at 85℃ for 1 h. Through precise control of environmental factors such as pH, extractant concentrations and O:A ratios, the process achieves exceptional separation efficiency for all metals in LIBs. The separation strategy employs: (i) a novel Primene JM-T/Cyanex 272 synergistic system that selectively extracts > 90 % of Co/Mn/Ni as M(L)2·2RNH3+SO42− (M = Co, Mn, Ni) complexes while leaving Li in raffinate; (ii) P204 extractant exhibiting exceptional Mn selectivity from Co/Ni (>99 % Mn extraction), with slope analysis confirming Mn(HL2)2 as the dominant species; and (iii) Cyanex 272 achieving > 98 % Co extraction (as Co(HL2)2) with minimal Ni co-extraction (<10 %). Besides, XPS, XRD, SEM-EDS analysis confirmed the achievement of high-purity (>98 %) precipitates of Li2CO3, MnCO3, Co(OH)2, and NiCO3. Futhermore, all extractants exhibit excellent reusability over 10 cycles. Thus, this study presents a lithium-first recovery strategy, with sequential separation and extraction of Mn, Co, and Ni, demonstrating both process efficiency and commercial potential for LIB recycling.
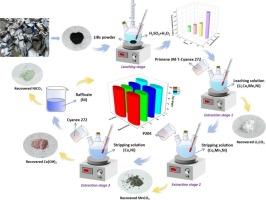
综合溶剂萃取沉淀法从废锂离子电池中序贯分离回收Li、Mn、Co和Ni
锂离子电池(LIB)生产中对锂和关键元素的需求不断增长,凸显了回收利用以支持低碳运输和能源储存的迫切需要。在这里,我们提出了一种集成的湿法冶金工艺,将优化的酸浸与多阶段分离级联相结合,从废LIB阴极中回收 >; 98 %的Li, Co, Mn和Ni。用3.0 M H2SO4和10 vol% H2O2,在85℃下,1 h,几乎可以定量地从LIB粉末中浸出Li、Co、Mn和Ni。通过精确控制环境因素,如pH值、萃取剂浓度和O:A比,该工艺对lib中的所有金属都实现了卓越的分离效率。分离策略采用:(i)一种新型Primene JM-T/Cyanex 272协同体系,选择性地提取 >; 90 %的Co/Mn/Ni为M(L)2·2RNH3+SO42−(M = Co, Mn, Ni)配合物,同时将Li留在萃余液中;(ii) P204萃取剂对Co/Ni (>;99 % Mn萃取)表现出优异的Mn选择性,斜率分析证实Mn(HL2)2是优势种;(iii) Cyanex 272实现 >; 98 %的Co萃取(以Co(HL2)2的形式),同时最少的Ni共萃取(<10 %)。此外,XPS、XRD、SEM-EDS分析证实了Li2CO3、MnCO3、Co(OH)2和NiCO3的高纯度(>98 %)沉淀的实现。此外,所有萃取剂在10个循环中都具有良好的可重复使用性。因此,本研究提出了锂优先回收策略,通过顺序分离和提取Mn, Co和Ni,展示了锂电池回收的工艺效率和商业潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


