CRISPR/Cas12a-Triggered DNA Windmill-Driven Dual-Mode Self-Powered Biosensor with Capacitor Signal Amplification for Ultrasensitive and Portable Detection of Thalassemia
IF 3.7
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
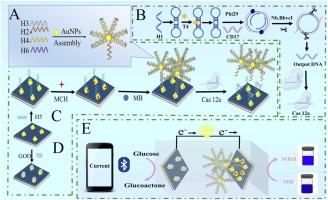
Herein, we report a CRISPR/Cas12a-triggered DNA windmill-driven dual-mode self-powered biosensor integrated with capacitor signal amplification for the ultrasensitive and portable detection of the thalassemia gene CD17. This innovative platform combines molecular recognition, nanoengineering, and portable electronics to overcome limitations in conventional thalassemia diagnosis. The DNA windmill nanostructure (DW), self-assembled from four DNA strands, provides abundant methylene blue (MB) binding sites for dual-signal amplification. CRISPR/Cas12a acts as a programmable molecular switch, activating trans-cleavage upon recognizing CD17-triggered RCA products, precisely regulating DW integrity and MB dissociation. The AuNPs/graphdiyne nanocomposite-modified bioelectrode significantly enhances electron transfer efficiency, achieving great current amplification through sp/sp2 hybrid carbon networks and plasmonic effects. By coupling enzyme-powered biofuel cells with a 1000 μF capacitor, the system converts biochemical signals into amplified electrical outputs while enabling Bluetooth-enabled smartphone readouts for field deployment. The dual-mode detection, integrating electrochemical and colorimetric channels, demonstrates ultra-low detection limits of 36.0 aM (electrochemical) and 52.1 aM (colorimetric) with a 5-log dynamic range (0.0001–10,000 pM). Clinical validation shows 96.5-108.6% recovery in human serum, supported by <5% RSD in reproducibility tests. This work pioneers CRISPR-powered portable diagnostics with CRISPR-windmill signal transduction and capacitor-boosted sensitivity, offering transformative potential for point-of-care genetic disease screening.
带有电容信号放大的CRISPR/ cas12a触发DNA风车驱动双模式自供电生物传感器用于超灵敏和便携式检测地中海贫血
在此,我们报道了一种CRISPR/ cas12a触发的DNA风车驱动双模式自供电生物传感器,集成了电容信号放大,用于超灵敏和便携式检测地中海贫血基因CD17。这个创新的平台结合了分子识别、纳米工程和便携式电子设备,克服了传统地中海贫血诊断的局限性。DNA风车纳米结构(DW)由四条DNA链自组装而成,为双信号扩增提供了丰富的亚甲基蓝(MB)结合位点。CRISPR/Cas12a作为一个可编程的分子开关,在识别cd17触发的RCA产物时激活反式切割,精确调节DW完整性和MB解离。AuNPs/石墨炔纳米复合材料修饰的生物电极显著提高了电子传递效率,通过sp/sp2杂化碳网络和等离子体效应实现了较大的电流放大。通过将酶驱动的生物燃料电池与1000 μF的电容器耦合,该系统将生化信号转换为放大的电输出,同时支持蓝牙智能手机读数,以供现场部署。双模式检测,集成了电化学和比色通道,超低检出限为36.0 aM(电化学)和52.1 aM(比色),5对数动态范围(0.0001-10,000 pM)。临床验证表明,在人血清中回收率为96.5-108.6%,在重复性试验中RSD为5%。这项工作开创了crispr驱动的便携式诊断技术,具有crispr风车信号转导和电容器增强的灵敏度,为即时遗传病筛查提供了变革潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sensors and Actuators B: Chemical
工程技术-电化学
CiteScore
14.60
自引率
11.90%
发文量
1776
审稿时长
3.2 months
期刊介绍:
Sensors & Actuators, B: Chemical is an international journal focused on the research and development of chemical transducers. It covers chemical sensors and biosensors, chemical actuators, and analytical microsystems. The journal is interdisciplinary, aiming to publish original works showcasing substantial advancements beyond the current state of the art in these fields, with practical applicability to solving meaningful analytical problems. Review articles are accepted by invitation from an Editor of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


