Non-cell-autonomous tumor promotion in DICER1 cancer predisposition
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
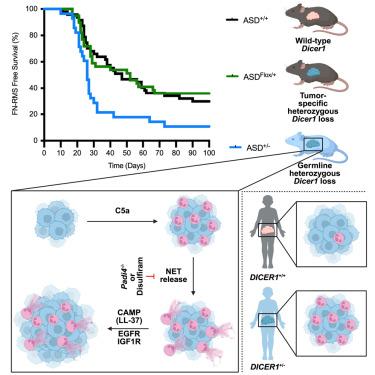
DICER1-related tumors are characterized by germline loss-of-function mutations in one DICER1 allele (DICER1+/−) and a somatic “second hit” mutation in the remaining DICER1 allele. Whether the germline DICER1+/− mutation participates in tumorigenesis is unknown. We show that germline heterozygous loss of Dicer1 promotes tumor formation via aberrant neutrophil function in spontaneous and allograft mouse models of rhabdomyosarcoma. Germline heterozygous deletion of Dicer1 decreased tumor latency and increased tumor penetrance, while conditional heterozygous deletion in tumor cells did not, illustrating that non-cell-autonomous contributions were required for tumor promotion. We show that Dicer1+/− murine and human tumors were enriched for neutrophils and that tumor-bearing mice had abundant circulating neutrophil extracellular traps (NETs). Genetically and pharmacologically preventing NET release reduced tumor promotion in Dicer1+/− mice, suggesting NETs promote tumor growth. These findings demonstrate that germline DICER1+/− mutations promote tumor growth and suggest that targeting neutrophils/NET release may reduce cancer risk in DICER1+/− individuals.
非细胞自主肿瘤促进DICER1癌症易感性
DICER1相关肿瘤的特征是一个DICER1等位基因(DICER1+/−)的种系功能丧失突变和其余DICER1等位基因的体细胞“二次打击”突变。种系DICER1+/−突变是否参与肿瘤发生尚不清楚。我们发现,在自发性和同种异体移植小鼠横纹肌肉瘤模型中,种系杂合缺失Dicer1通过异常中性粒细胞功能促进肿瘤形成。种系杂合缺失Dicer1会降低肿瘤潜伏期,增加肿瘤外显率,而肿瘤细胞中的条件杂合缺失则不会,说明肿瘤促进需要非细胞自主贡献。我们发现Dicer1+/−小鼠和人类肿瘤富含中性粒细胞,荷瘤小鼠具有丰富的循环中性粒细胞胞外陷阱(NETs)。从遗传学和药理学上阻止NET的释放可减少Dicer1+/−小鼠的肿瘤促进,表明NET促进肿瘤生长。这些发现表明生殖系DICER1+/ -突变促进肿瘤生长,并提示靶向中性粒细胞/NET释放可能降低DICER1+/ -个体的癌症风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


