Phototransformation of benzodiazepines in water mediated by dissolved black carbon: Mechanisms and the structure-reactivity relationship
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
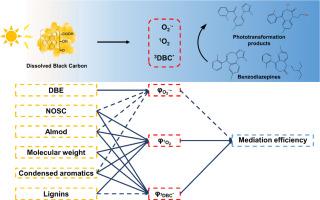
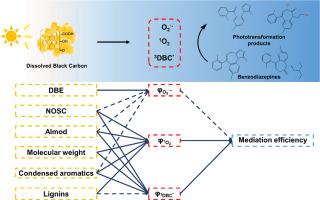
Dissolved black carbon (DBC) within aquatic dissolved organic matters demonstrates potent photochemical activity, yet its effects on emerging contaminant transformation and structure-reactivity relationships remain inadequately characterized. In this study, benzodiazepines were employed as model emerging contaminants to investigate the photoactivity, mediation effect, and structural-activity relationships of five DBC samples and four well-studied dissolved humic substances (DHSs) under simulated sunlight irradiation. DBC efficiently generated superoxide anions (O2·-), singlet oxygen (1O2), and triplet excited states, thereby facilitating the phototransformation of benzodiazepines. Under DBC mediation, midazolam and flurazepam achieved transformation rates of 61.1-99.5% and 19.0-84.6%, respectively, within 8 h, exceeding direct phototransformation efficiencies (1.3-13.3%). Triplet excited states were identified as the dominant reactive intermediates, contributing more than 46.7% to the photoreactions. At equivalent total organic carbon levels, DBCs averagely exhibited 1.3-, 2.7-, and 3.3-fold higher photoactivity (O₂·-, ¹O₂, and triplet excited states) alongside 2.5- and 4.7-fold enhanced benzodiazepine (midazolam and flurazepam) transformation compared to DHSs based on quantum yield measurements. Phototransformation pathways of benzodiazepines via DBC-mediation included ¹O₂ oxidation and charge transfer with triplet excited states, generating charge-separated intermediates that subsequently induce ring cleavage and coupling reactions. The molecular structures of DBC and DHS were characterized using UV-visible spectroscopy and Fourier transform-ion cyclotron resonance mass spectrometry. To elucidate the structure-reactivity relationship, Spearman rank correlation analysis, structural equation modeling, and orthogonal partial least squares regression were employed. The results identify molecular weight, aromaticity, and oxidation degree as key structural determinants of photoactivity. Low-molecular-weight compounds containing condensed aromatic structures and lignins demonstrate superior photoactivity in DBC/DHS structures, driving triplet excited-state and ¹O₂ formation, while O2·- might be generated from different structures. This study elucidated DBC mediation mechanisms and structure-reactivity relationships in benzodiazepine phototransformation, identifying low-molecular-weight fractions containing condensed aromatic and lignin moieties as dominant photoactive drivers in aquatic environment.
溶解黑碳介导苯二氮卓类药物在水中的光转化:机理和结构-反应关系
溶解黑碳(DBC)在水生溶解有机物中表现出强大的光化学活性,但其对新出现的污染物转化和结构-反应关系的影响仍未得到充分的表征。本研究以苯二氮卓类药物为模拟新兴污染物,研究了5种DBC样品和4种已研究过的溶解腐殖质(DHSs)在模拟阳光照射下的光活性、中介作用和构效关系。DBC有效地生成了超氧阴离子(O2·-)、单重态氧(1O2)和三重态激发态,从而促进了苯二氮卓类药物的光转化。在DBC的作用下,咪达唑仑和氟西泮在8 h内的转化率分别为61.1 ~ 99.5%和19.0 ~ 84.6%,超过了直接光转化效率(1.3 ~ 13.3%)。三态激发态是主要的反应中间体,对光反应的贡献超过46.7%。在相同的总有机碳水平下,DBCs平均表现出1.3倍、2.7倍和3.3倍的光活性(O₂·-、¹O₂和三重态激发态),以及2.5倍和4.7倍的苯二氮卓类(咪达唑仑和氟拉西泮)转化。苯二氮卓类药物在dbc介质下的光转化途径包括三态激发态的1 O 2氧化和电荷转移,产生电荷分离的中间体,从而诱导环裂解和偶联反应。利用紫外可见光谱和傅里叶变换离子回旋共振质谱对DBC和DHS的分子结构进行了表征。采用Spearman秩相关分析、结构方程模型和正交偏最小二乘回归等方法对结构-反应性关系进行分析。结果确定分子量,芳香性和氧化度是光活性的关键结构决定因素。含有缩合芳香族结构和木质素的低分子量化合物在DBC/DHS结构中表现出优异的光活性,驱动三重态激发态和¹O₂的形成,而O2·-可能由不同的结构产生。本研究阐明了DBC在苯二氮卓类药物光转化中的中介机制和结构-反应性关系,确定了含凝聚芳香族和木质素的低分子量组分是水生环境中主要的光活性驱动因子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


