Carbonate radicals in environmental systems: mechanistic insights and engineering applications
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
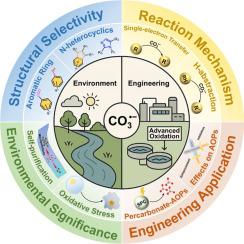
Carbonate radical (CO3•−) is prevalent in both natural environments and engineered water treatment systems, originating from reactions between carbonate species (CO32−/HCO3−) and free radicals or triplet states of dissolved organic matter (3DOM*). Its significant roles in environmental oxidation, advanced oxidative water treatment, and cellular oxidative damage are increasingly recognized. Despite extensive research on CO3•−, a gap remains in comprehensive reviews addressing its kinetics and mechanisms in pollutant removal and environmental impact. This review synthesizes previous studies, tracing the historical recognition of CO3•− in environmental sciences and comparing various detection methods. The reaction rate constants of CO3•− with organic contaminants (kCO3•−) are summarized, and the relationship between pollutant structure and reactivity is explored. Reaction mechanisms and the selectivity of CO3•− are critically evaluated in comparison to other common radicals, such as hydroxyl (HO•) and sulfate (SO4•−). Furthermore, the dual roles of CO3•− as both primary and secondary radicals in advanced oxidation processes (AOPs), along with its impact on degradation pathways and byproduct toxicity, are assessed. Finally, the environmental implications of CO3•−, including its contributions to self-purification, metal transport, and biological oxidative damage, are discussed. This review aims to offer a comprehensive framework for understanding CO3•− in environmental contexts, enhancing its application in AOP systems and natural remediation.
环境系统中的碳酸盐自由基:机械见解和工程应用
碳酸盐自由基(CO3•−)在自然环境和工程水处理系统中都很普遍,起源于碳酸盐物种(CO32−/HCO3−)与自由基或溶解有机物(3DOM*)的三重态之间的反应。它在环境氧化、高级氧化水处理和细胞氧化损伤等方面的重要作用日益被人们所认识。尽管对CO3•−进行了广泛的研究,但在解决其污染物去除和环境影响的动力学和机制方面仍存在空白。本文综述了以往的研究,追溯了环境科学中对CO3•−的历史认识,并比较了各种检测方法。综述了CO3•−与有机污染物(kCO3•−)的反应速率常数,探讨了污染物结构与反应性的关系。与其他常见自由基,如羟基(HO•)和硫酸盐(SO4•−)相比,对CO3•−的反应机制和选择性进行了严格的评估。此外,CO3•−在高级氧化过程(AOPs)中作为主要和次要自由基的双重作用,以及其对降解途径和副产物毒性的影响,进行了评估。最后,讨论了CO3•−对环境的影响,包括其对自净化、金属运输和生物氧化损伤的贡献。本综述旨在提供一个全面的框架来理解环境背景下的CO3•−,增强其在AOP系统和自然修复中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


