Design, Synthesis, and Biological Profiling of Novel Aryl-Spirocyclic Diamine Derivatives with Potential Antidepressant-like Properties
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
To meet the clinical need for therapeutic efficacy against cognitive impairment and pain comorbidities in depression, a rational drug design approach was used in the current study to synthesize a series of spirocyclic diamine derivatives. Among them, (R)-D24 exhibited triple monoamine reuptake inhibitory activity (SERT, NET, and DAT), while D6 and D17 showed potent inhibitory activity against both SERT and 5-HT3AR. D6 and D17 have acceptable systemic exposure and oral bioavailability in mice and low clearance in human liver microsomes. D6 and D17 exhibited favorable safety profiles in hepatic, renal, and hERG toxicity assays and showed potent antidepressant-like effects in the forced swim test, supporting their potential for further preclinical investigation. In addition, the compound-target interactions of the key compounds were analyzed through molecular docking. These results highlight the rationality of our design and provide new perspectives for the development of antidepressant drugs.
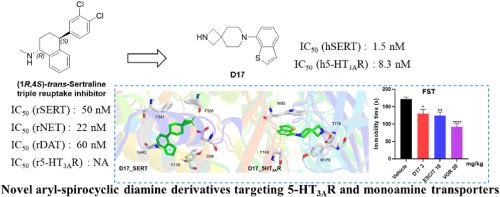
具有潜在抗抑郁样特性的新型芳基-螺环二胺衍生物的设计、合成和生物学分析
为满足临床对抑郁症认知功能障碍和疼痛共病治疗效果的需要,本研究采用合理的药物设计方法合成了一系列螺环二胺衍生物。其中(R)-D24表现出三重单胺再摄取抑制活性(SERT、NET和DAT),而D6和D17对SERT和5-HT3AR均表现出较强的抑制活性。D6和D17在小鼠体内具有可接受的全身暴露和口服生物利用度,在人肝微粒体中的清除率较低。D6和D17在肝、肾和hERG毒性试验中表现出良好的安全性,并在强迫游泳试验中显示出有效的抗抑郁样作用,支持其进一步临床前研究的潜力。此外,通过分子对接分析了关键化合物的化合物-靶标相互作用。这些结果突出了我们设计的合理性,并为抗抑郁药物的开发提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


