Electrocatalytic Dehydrogenative Lactonization of Benzylic Alcohols: A Sustainable Access to Phthalides via N-hydroxyphthalimide Mediation
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
A sustainable and efficient electrochemical method for the direct oxidative lactonization of benzylic alcohols, enabling rapid access to isobenzofuran-1(3H)-ones (phthalides) is presented. This electrocatalytic transformation leverages N-hydroxyphthalimide as a redox mediator under mild, metal-free conditions, offering an environmentally friendly alternative to traditional oxidation protocols. The method demonstrates broad substrate scope and delivers phthalide derivatives consistently in good to excellent yields. Mechanistic studies, combining cyclic voltammetry and density functional theory calculations, support a radical-mediated hydrogen atom transfer mechanism driven by phthalimide-N-oxyl radicals. Importantly, the utility of the protocol extends beyond model substrates: it is successfully applied to the synthesis of pharmaceutically relevant compounds, including talopram and a key intermediate for a neuropeptide Y5 receptor antagonist. Overall, this work underscores the power of electrosynthesis in modern organic chemistry, merging green chemistry principles with synthetic efficiency.
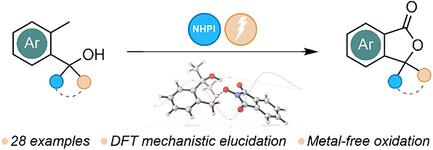
苯甲酸醇的电催化脱氢内酯化:通过n -羟基邻苯二甲酸亚胺介导的可持续获取邻苯二甲酸酯
提出了一种可持续的、高效的苯基醇直接氧化内酯化的电化学方法,可以快速获得异苯并呋喃-1(3H)- 1(邻苯二甲酸酯)。这种电催化转化利用n -羟基邻苯二胺作为氧化还原介质,在温和、无金属的条件下,为传统氧化方案提供了一种环保的替代方案。该方法证明了广泛的底物范围,并提供了苯酞衍生物一致良好的收率。结合循环伏安法和密度泛函理论计算的机理研究支持了邻苯二胺- n -氧自由基驱动的自由基介导的氢原子转移机制。重要的是,该方案的实用性超出了模型底物:它成功地应用于药学相关化合物的合成,包括他洛普兰和神经肽Y5受体拮抗剂的关键中间体。总的来说,这项工作强调了电合成在现代有机化学中的力量,将绿色化学原理与合成效率相结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


