Enhanced gold recovery from e-waste by biological thiosulfate leaching and trace gold extraction with UiO-66-NH2
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The recycling of precious metals from electronic waste (e-waste) is essential to respond to the increasing demand for strategic metal. Compared to the cyanide method, the thiosulfate bioleaching method is considered a greener and cleaner approach for gold recovery. However, gold recovery from thiosulfate bioleaching solutions remains a challenge. This study addressed these challenges by optimizing the production of biogenic thiosulfate from Acidithiobacillus thiooxidans using a combination of NaN₃ and KCN at the optimal ratio of 4:1.The thiosulfate bioleaching process achieved a 30% extraction efficiency (0.6 mg/L) of gold, from 10 g/L of spent printed circuit boards. The adsorption of gold was explored using UiO-66-NH2, a highly porous metal–organic framework (MOF) with a Brunauer–Emmett–Teller (BET) surface area of 1840 m2/g and a pore size of 5–15 nm. Fourier Transform Infrared Spectroscopy (FTIR) analysis confirmed the presence of amino functional groups, enhance the gold-binding affinity. The synthesized metal–organic framework, UiO-66-NH2, demonstrated a 50% gold adsorption capacity from the bioleaching solution within 2 h. The electrostatic attraction between the NH3+ groups of UiO-66-NH2 and , combined with internal Zr-OH complexes, played a crucial role in the adsorption mechanism. Gold ions (Au3+) were reduced to elemental gold (Au0) via an oxidation–reduction reaction facilitated by amino groups, as confirmed through FTIR, field emission scanning electron microscopy (FESEM), mapping and energy-dispersive X-ray spectroscopy (EDS) analysis. Adsorption kinetics analysis indicated that the process followed a pseudo-second-order model. These results demonstratea sustainable method for recovering gold directly from e-waste bioleaching solutions.
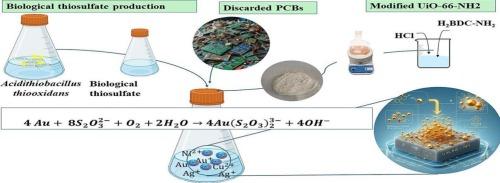
生物硫代硫酸盐浸出和UiO-66-NH2萃取微量金提高电子垃圾中金的回收率
从电子废物(电子废物)中回收贵金属对于应对日益增长的战略金属需求至关重要。与氰化法相比,硫代硫酸盐生物浸出法被认为是一种更环保、更清洁的金回收方法。然而,从硫代硫酸盐生物浸出溶液中回收金仍然是一个挑战。该研究通过以4:1的最佳比例使用NaN₃和KCN的组合,优化了从硫氧化硫硫杆菌生产生物源硫代硫酸盐的方法,解决了这些挑战。硫代硫酸盐生物浸出工艺从10 g/L的废印刷电路板中提取金的效率为30% (0.6 mg/L)。研究了uuo -66- nh2对金的吸附作用。uuo -66- nh2是一种高孔金属有机骨架(MOF),其比表面积为1840 m2/g,孔径为5 ~ 15 nm。傅里叶变换红外光谱(FTIR)分析证实了氨基官能团的存在,增强了金的结合亲和力。合成的金属有机骨架UiO-66-NH2在2 h内对生物浸出液中的金具有50%的吸附能力。UiO-66-NH2的NH3+基团与AuS2O323-之间的静电吸引以及内部的Zr-OH配合物在吸附机理中起关键作用。通过红外光谱(FTIR)、场发射扫描电镜(FESEM)、能谱分析和能谱分析(EDS)证实,金离子(Au3+)通过氨基促进的氧化还原反应被还原为元素金(Au0)。吸附动力学分析表明,该过程符合准二阶模型。这些结果证明了直接从电子垃圾生物浸出溶液中回收黄金的可持续方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


